Relay®Pro
Uniquely Inspired for Ideal Placement
At Terumo Aortic, we follow your lead. That’s why our Relay® family of devices was adapted for excellent performance in every patient’s thoracic aorta.
Inspiring Confidence with Next-Generation Device Technology
The RelayPro Thoracic Stent-Graft System is intended to treat various thoracic aortic pathologies in adult patients.

Fabric
- Low profile
- High strength
- Low permeability
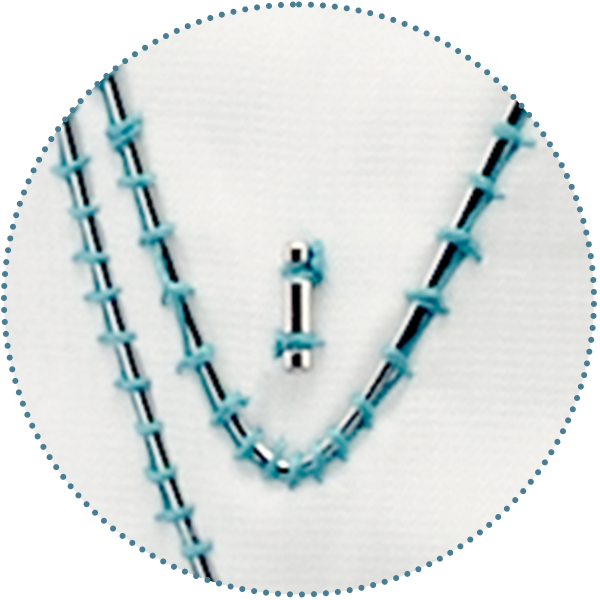
Suture
5-0 braided polyester surgical
suture impregnated with PTFE:
- High wear resistance
- High tensile strength
Stents
Electropolished Nitinol:
- Super-elastic properties
- Proven fatigue endurance
Key Features
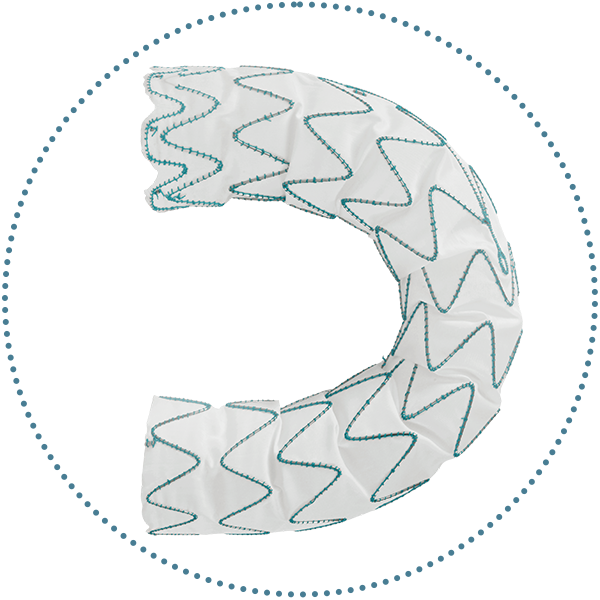
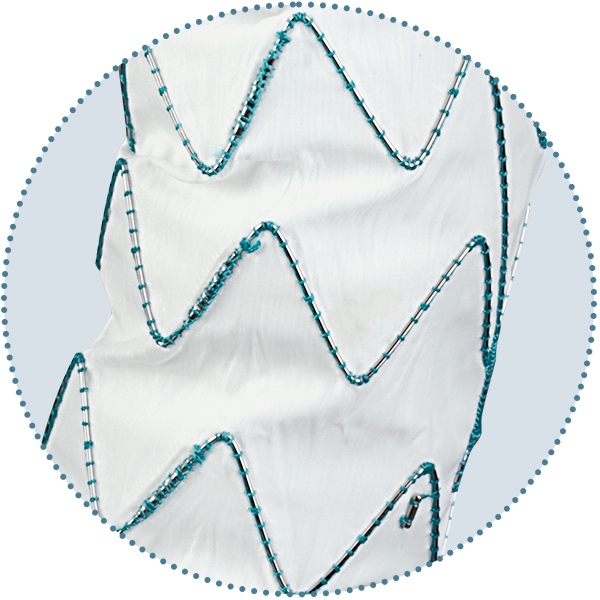
Graft
- Multiple Size Options
- Performance Zones
- Proximal End Configuration
- S-Bar Technology

Delivery System
- Dual Sheath Technology
- Pre-Curved Inner Catheter
- Low Profile Delivery System
- NBS: Support Wires & Flared End
- NBS: Asymmetrical Proximal Clasping
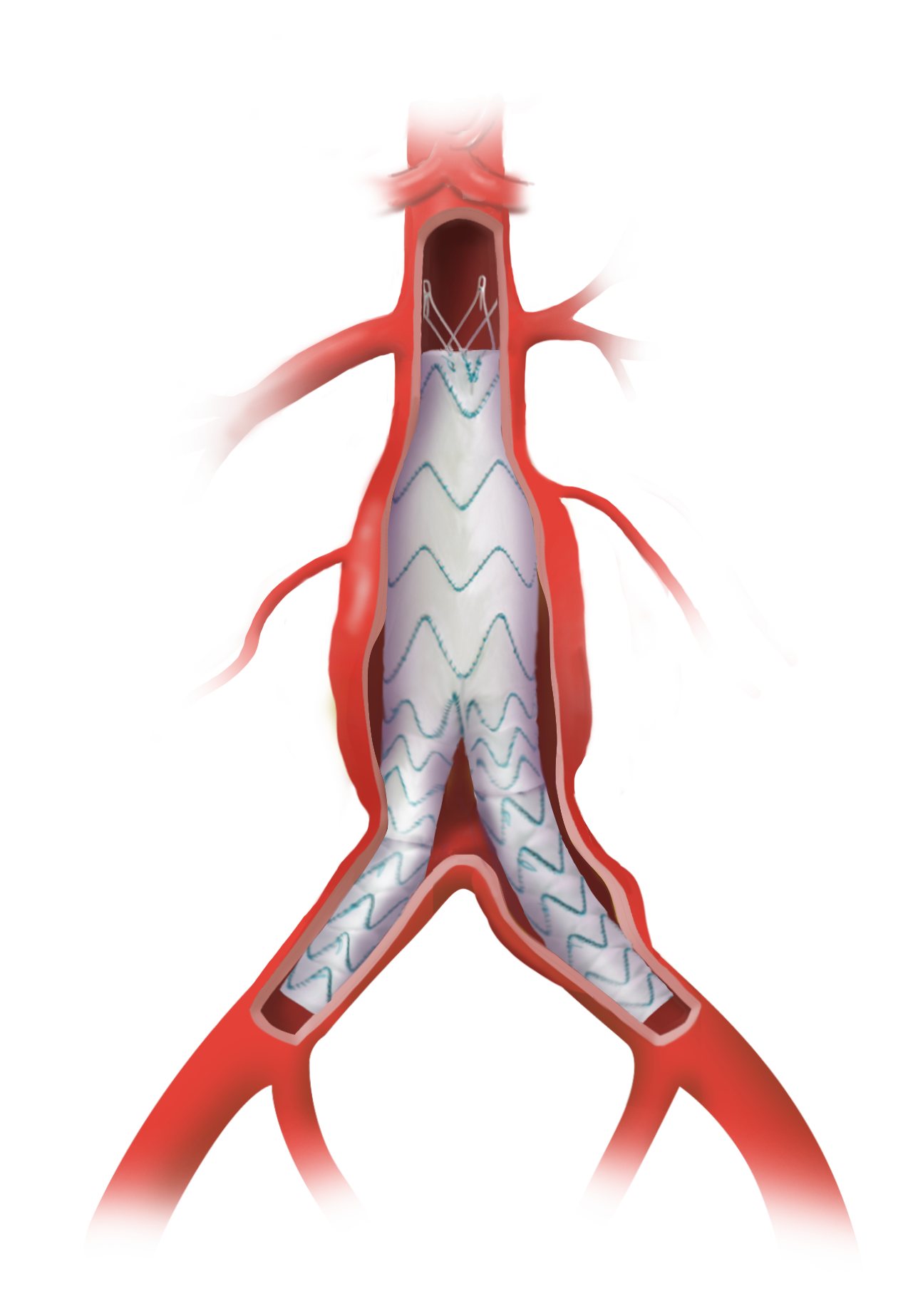
Multiple Size Options for a Personalised Approach
The standard portfolio has a wide range of sizes and tapers, allowing each patient access to the right solution, every time.
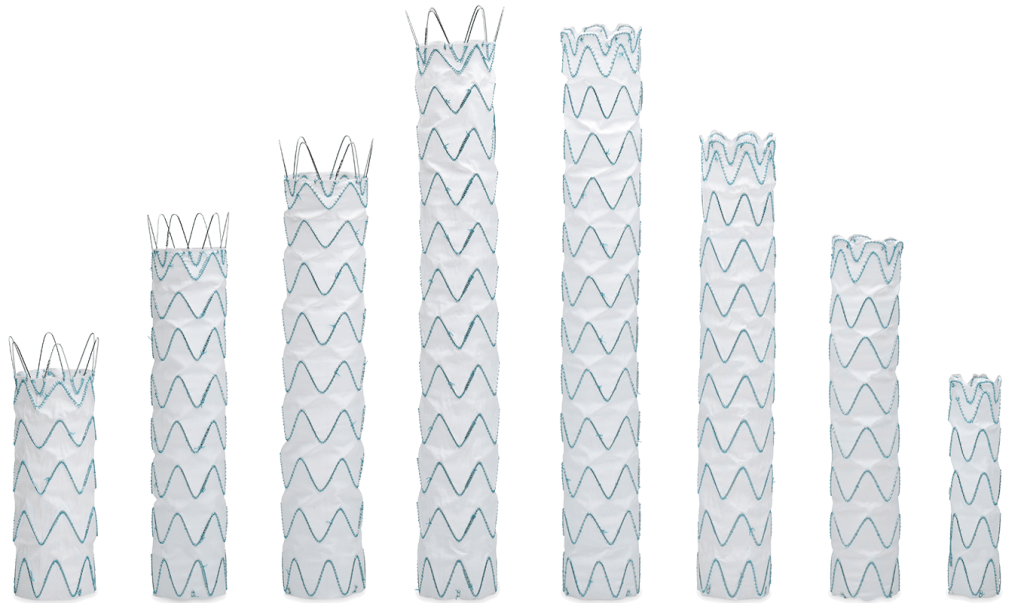
One of the key benefits of RelayPro is being able to choose from a range of proximal configurations allowing me to tailor my device selection to meet the individual needs of each patient.2
Venkatesh G. Ramaiah, M.D.
Designed to Respect the Thoracic Anatomy
The RelayPro stent graft is divided into performance zones. Each zone is designed to serve a specific purpose and therefore distributes an appropriate radial load independent of other zones.
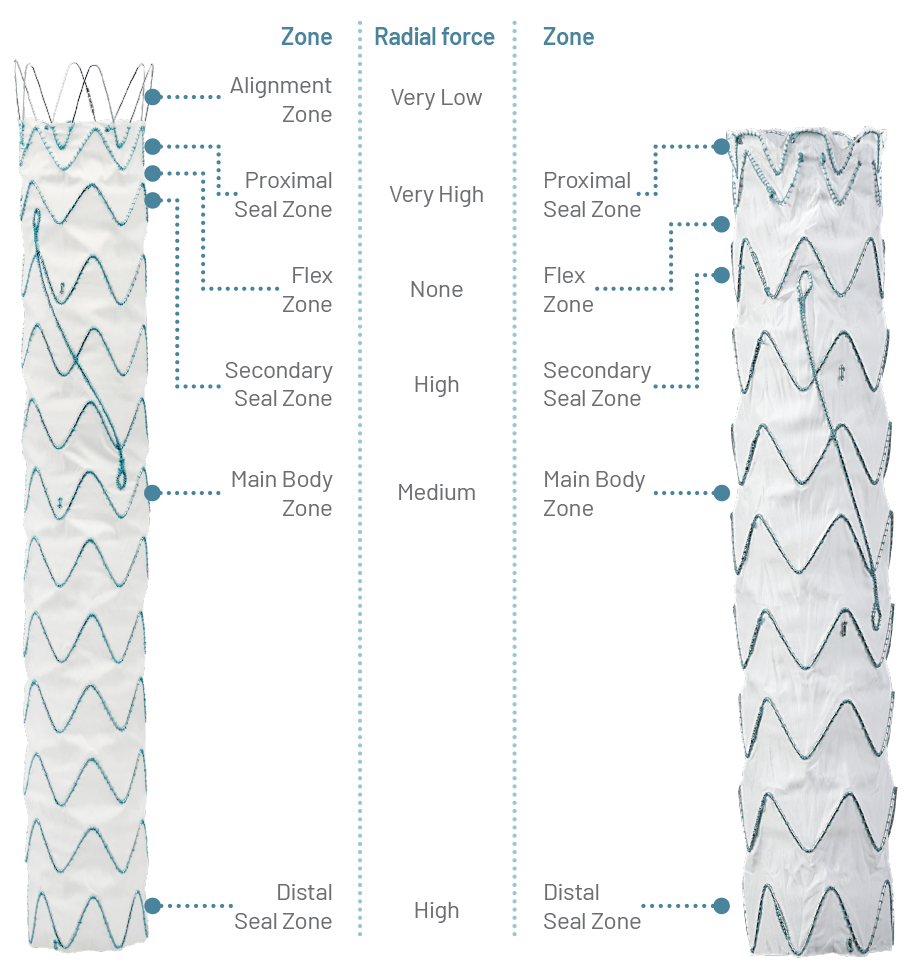
Uniform Sealing and Secure Fixation
Both RelayPro Bare Stent and Non Bare Stent offer multiple sealing points.

Bare Stent Configuration
Partial overlapping of the bare stent with the first covered stent to maximise the number of sealing points.

Non Bare Stent Configuration
A crown-shaped nitinol stent overlapping with the proximal sealing stent, both covered with fabric, designed to maximise conformability and minimise infolding.
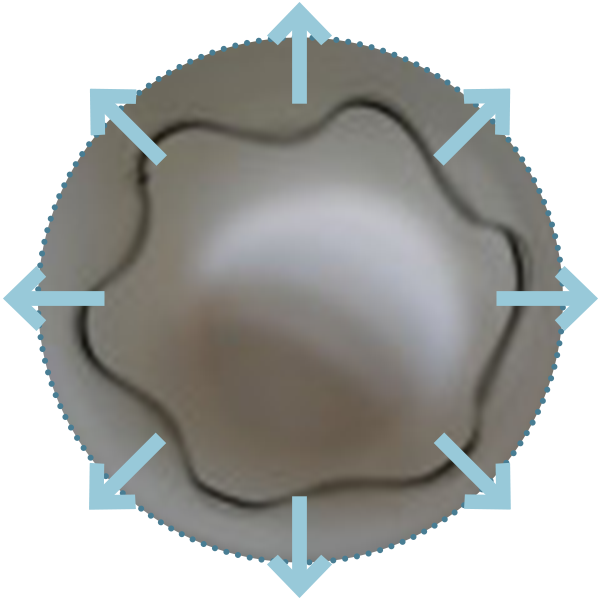
High Radial Load
Both proximal configurations are designed to deliver high radial load for an effective apposition and fixation of the graft against the aortic wall.
Ahead of the Curve with the S-Bar Technology
S-Bar, an s-shaped nitinol wire, intended to provide columnar strength to the endograft and to enhance conformability by adapting to the natural curvature of the aorta.
Shortened length to optimise the treatment in tortuous aortas, enabling the more distal portion of the graft to flex in any direction.
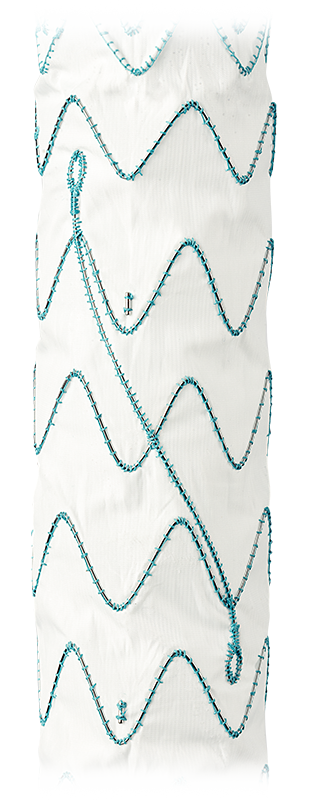
RelayPro NBS: Designed to minimise Bird-beaking and Retroflex
RelayPro NBS, the only thoracic endograft available on the market with a Non-Bare Stent configuration that can be used as a standalone proximal component.
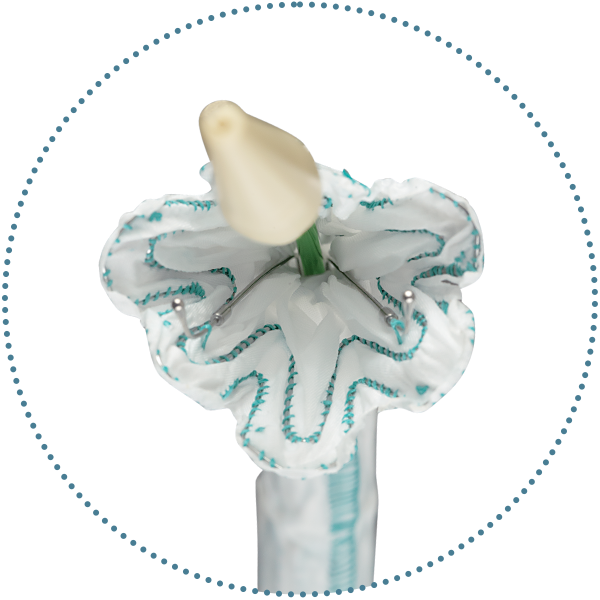
Minimises the risk of retroflex
During deployment, two support wires* guide the inferior portion toward the inner aortic wall, keeping it aligned with the landing zone, minimising the risk of retroflex

Ability to reposition
Two clasped stent apices, both located on the outer curve of the RelayPro NBS, for a precise and controlled deployment, preserving the ability to reposition

Minimises the risk of birdbeak
The Flared End configuration of the inner sheath enables partial expansion to improve the proximal alignment and precision for a correct apposition on the inner curve, minimising bird-beaking
Accurate deployment with favorable apposition even in hostile aortic arches contributed to low rates of early and midterm complications 3 ^
Hazem El Beyrouti, M.D.
* Support wires are only present in devices with 32mm or greater proximal stent-graft diameters.
^This study included all RelayPro configurations with the NBS being predominant. The RelayPro is not indicated for erosion and rupture and the FDA-approved device is further not indicated for IMH and only intended for the DTA. Approved indications will vary by region, always consult the corresponding IFU.
Deployment System
Precise, Accurate, and Controlled to Navigate the Arch with Care.
RelayPro’s ability to land accurately combined with its low profile will allow me to successfully treat complex anatomy with precision.4
Wilson Y. Szeto, M.D.
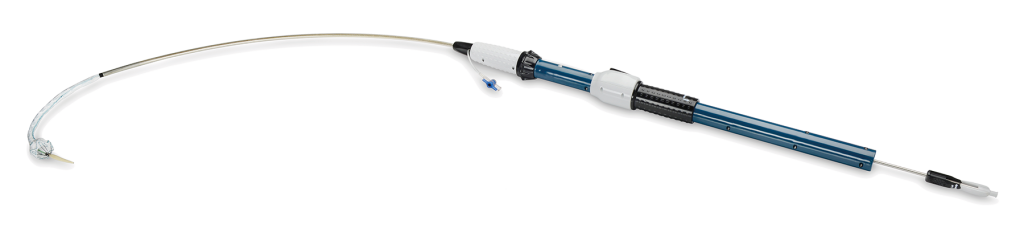
Proximal Clasping
- Allows for repositioning of the device and facilitates perpendicular deployment
Pre-Curved Inner Catheter
- Conforms to the aortic arch designed for alignment of the stent-graft
Soft Inner Sheath
- 30cm length
- Designed to provide navigability and to ensure accurate deployment, minimising trauma to surrounding anatomy
Coiled Outer Sheath
- 60cm length
- Designed to provide pushability and support during the advancement and manoeuvring through access vessel
Controller
- Allows for staged deployment enhancing control and accuracy in stent-graft placement
Mechanical Advantage
- Forward and backward gear system allows for small incremental movements of the stent-graft enhancing controlled delivery
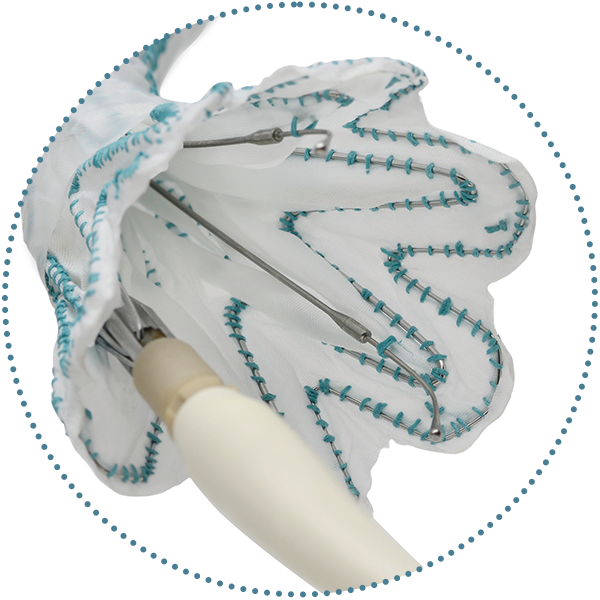
Non Bare Stent Configuration
Two clasped stent apices, both located on the outer curve of the RelayPro NBS

Bare Stent Configuration
Variable bare stent heights depending on proximal diameter
RelayPro's ability to navigate smoothly over the arch as a result of the Dual Sheath system enables accurate deployment […].2
Venkatesh G. Ramaiah, M.D.
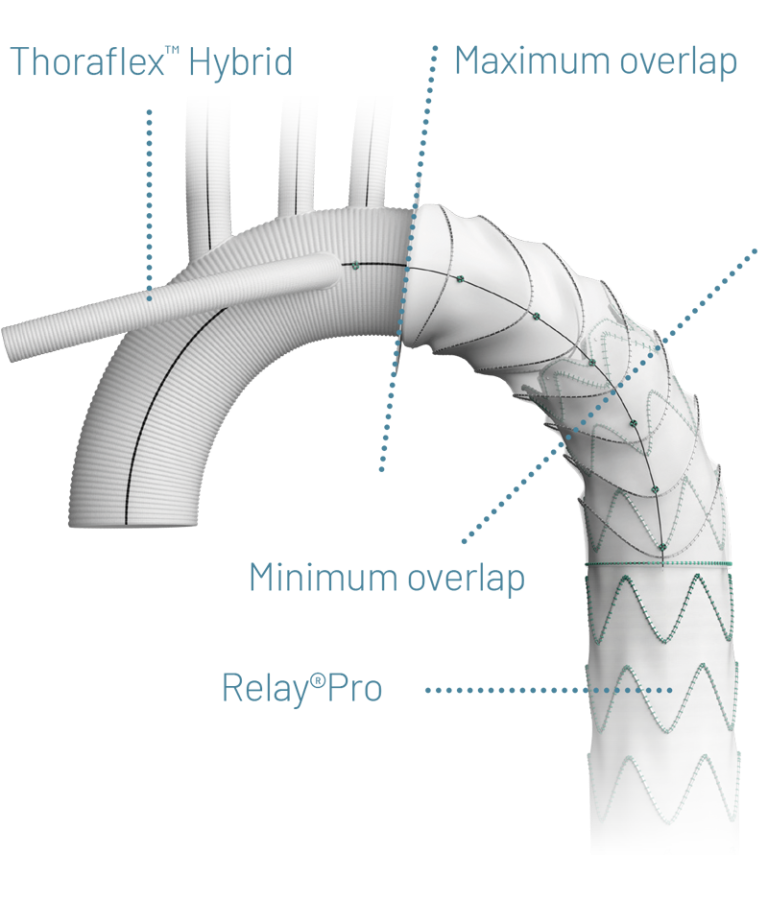
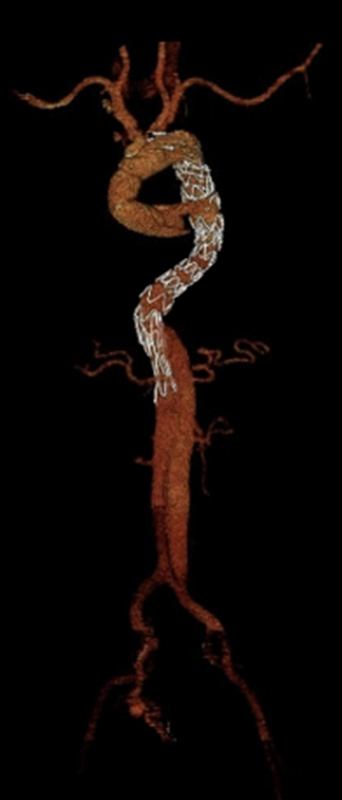
Thinking Ahead with On-Label Endovascular Extension with Thoraflex™ Hybrid
If the lesion requires use of a distal extension, only a Relay NBS configuration should be used:
- 44 year old patient
- Acute type A aortic dissection repaired with Thoraflex Hybrid, extended with RelayPro NBS thoracic stent graft system
With up to 33% of FET repairs needing a future aortic reintervention, on-label endovascular extension using RelayPro with Thoraflex Hybrid, is indicated to complete the downstream repair * 5
* Reinterventions included endovascular, open surgery or hybrid approaches. Note the Thoraflex Hybrid is ONLY indicated for treatment in cases of aneurysm and/or dissection. The CE-marked RelayPro is contraindicated in patients with connective tissue disorders.
Please refer to the device IFUs for complete indications, contraindications, warning’s and precautions.

Key Study
2021: FDA approval for Aneurysm, Penetrating Atherosclerotic Ulcer (P200045)
One-Year Results with a Low-Profile Endograft in Subjects with Thoracic Aortic Aneurysm and Ulcer Pathologies 6
Objective
Evaluation of safety and effectiveness of Relay®Pro for the treatment of descending thoracic aortic aneurysms or penetrating atherosclerotic ulcers.
Study Design
36 Centers
25 in USA, 11 in Japan
110 Patients
68 in USA, 42 in Japan
74.9 + 8.3
Mean Age (Years)
91%
9%
82.7%
Results
Technical success through 24 hours
110/110
Patients treated with a percutaneous femoral approach in the US Cohort
50/68
Stroke rate at 1 Year
4/110
Type Ia endoleak at 1 year
2/110
Absence of aneurysm expansion at 1 year
109/110
Absence of secondary intervention at 1 year
104/110
Conclusion
Relay®Pro demonstrated satisfactory 30-day safety and 1-year effectiveness for the treatment of patients with aneurysms of the DTA and PAUs.

Catriona was implanted with our RelayPro, Thoracoflo and Thoraflex Hybrid devices by Prof. Dr. Sabine Wipper. She got to meet two of our associates, Donna Abbott and Grant MacDougall, who had a hand in sewing her devices.
Downloads
Features & Benefits
RelayPro
Discover how each of the key features and benefits are integrated into our surgical portfolio to ensure the highest quality and performance possible.

References
Riambau et al. (2019). Prospective Multicenter Study of the Low-Profile Relay Stent-Graft in Patients with Thoracic Aortic Disease: The Regeneration Study. Annals of Vascular Surgery.
Wilson Y. Szeto, MD. Chief, Division of Cardiovascular Surgery. Hospital of the University of Pennsylvania-Penn Presbyterian. https://evtoday.com/news/fda-approves-terumo-aortic-relaypro-thoracic-stent-graft
El Beyrouti et al. (2020). Early results of a low-profile stent-graft for thoracic endovascular aortic repair. PLOS ONE
Venkatesh Ramaiah, MD, Chief of Complex Vascular Services and Network Director of Vascular Services of the HonorHealth hospital system, Scottsdale, Arizona
https://evtoday.com/news/terumo-aortic-completes-enrollment-of-relaypro-united-states-pivotal-trialKreibich et al. (2020). Aortic reinterventions after the frozen elephant trunk procedure. The Journal of Thoracic and Cardiovascular Surger
Szeto et al. (2022). One-Year Results with a Low-Profile Endograft in Subjects with Thoracic Aortic Aneurysm and Ulcer Pathologies. The Journal of Thoracic and Cardiovascular Surgery
Product Disclaimer
Product availability subject to regulatory approval.
An EU Declaration of Conformity may be requested from regulatoryaffairsuk@terumoaortic.com
Instructions for Use
View the eIFU for more information on use, indications, contraindications, warnings/precautions and availability within your market.
Contact us
Click the button below to get in touch. For more updates, follow us on X and LinkedIn. You can also view our VuMedi channel.