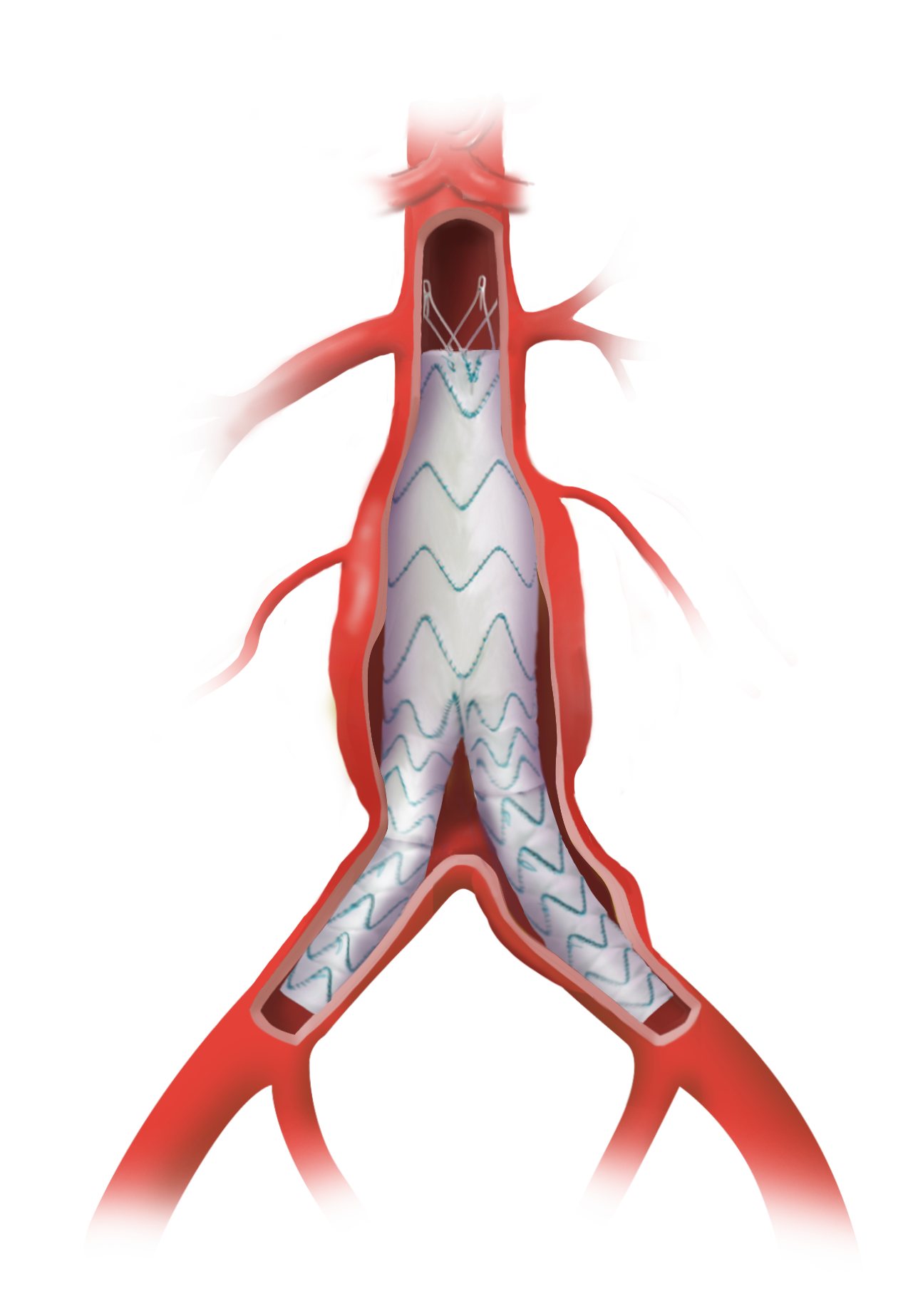
Portfolio
Products for Aortic Repair
Products
Product availability subject to local regulatory approval.
Products
Product availability subject to local regulatory approval.
Working together to address each patients’ aorta
Our aortic solutions range from Surgical, Endovascular to Hybrid, complementing each surgeon’s precise technique.
Surgical
Endovascular
Hybrid
Like you, we are 100% focused on the aorta, from the aortic root to the iliac arteries. We offer you the resources, partnership and confidence you need to decide on the best solutions for your patients’ diverse aortic challenges.
Whether it’s an off-the-shelf or custom-made product, our broad portfolio of diverse surgical, hybrid and endovascular solutions is designed to offer options as individualised as your patient’s anatomy.* With our wide range of aortic products, we enable you to find the best approach for each case — and each patient.
*Product availability and custom-made configurations are subject to local regulatory approval. Surgical graft portfolio (Gelweave and Gelsoft Plus) are not available as custom-made devices. Please contact customsolutions@terumoaortic.com or visit the Custom Solutions page for more information on available custom-made solutions.
Product Disclaimer
Product availability subject to regulatory approval.
An EU Declaration of Conformity may be requested from regulatoryaffairsuk@terumoaortic.com
Instructions for Use
View the eIFU for more information on use, indications, contraindications, warnings/precautions and availability within your market.
Discover our technology
Our products are powered by technologies that help ensure that every product you use can be implanted or deployed safely.