TREO®
Versatile by Design.
Fit for any Anatomy.*
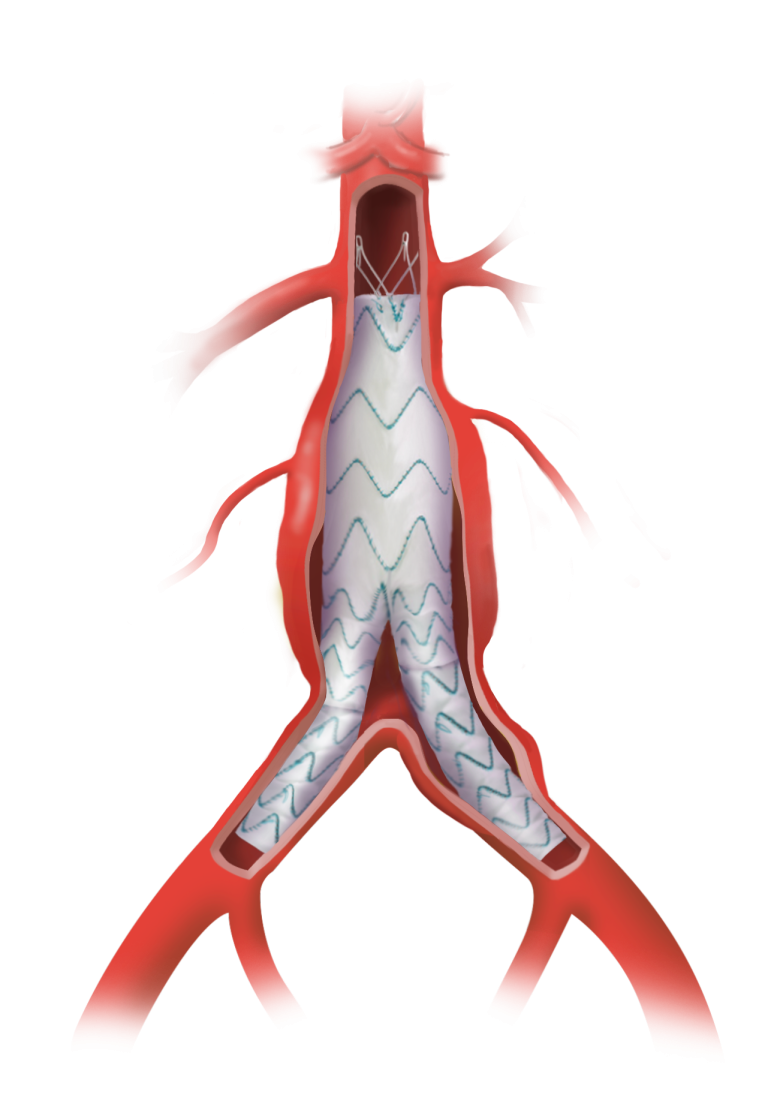
TREO® provides a wide range of endovascular device configurations to address the abdominal anatomy of each individual patient.
*Per IFU
TREO®
Repair. Remodel. Results.
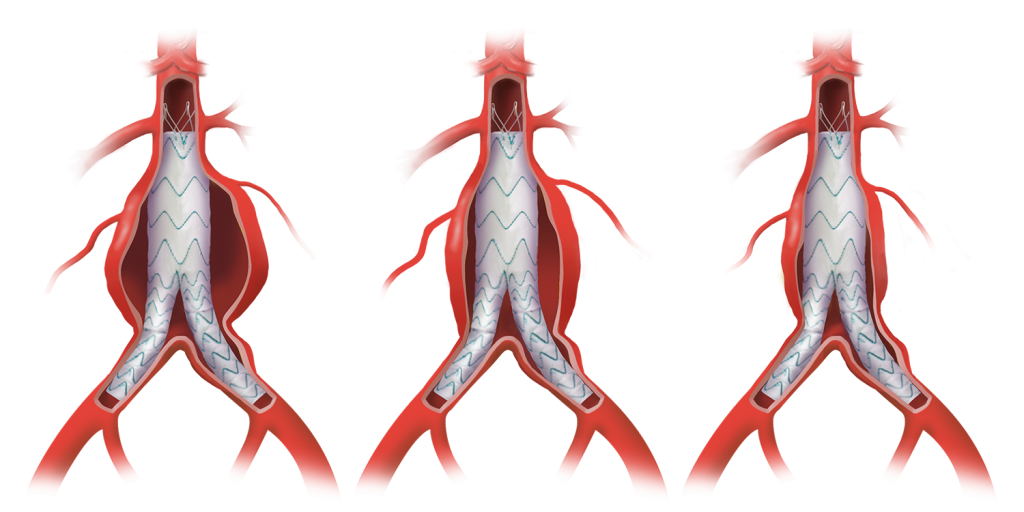
Learn how the TREO® abdominal stent-graft system supports EVAR success with sac regression, and aortic remodelling for improved outcomes.
Certainty simplified. And delivered.
Every aspect of the TREO Abdominal Stent-Graft System is engineered for performance.
- Two levels of confident fixation
- Lock stent technology works to prevent modular disconnection
- Unique leave-behind sheath to simplify the procedure
- Thousands of configurations to accommodate breadth of patients

Engineered for a personal approach
Every feature of TREO contributes to ideal performance in your endovascular procedure — and your patient’s abdominal aorta.
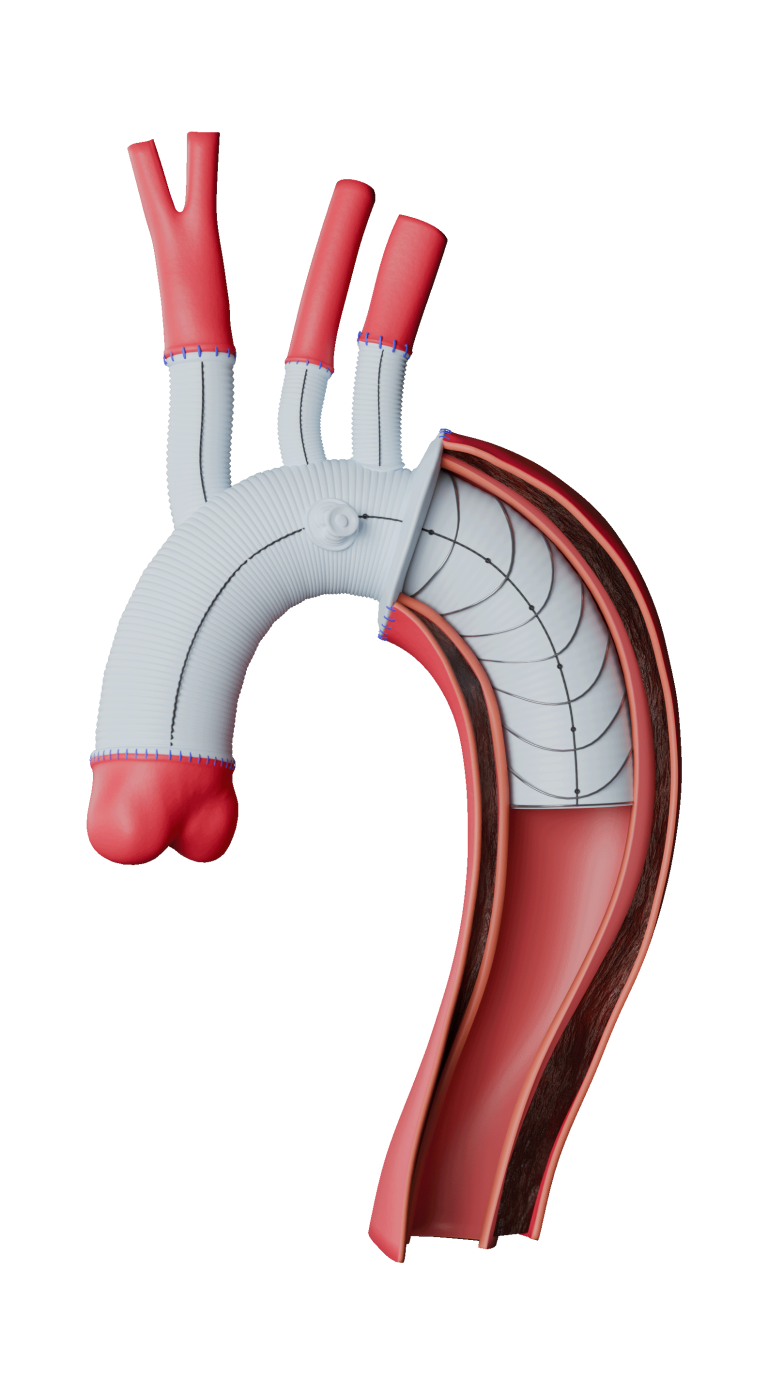
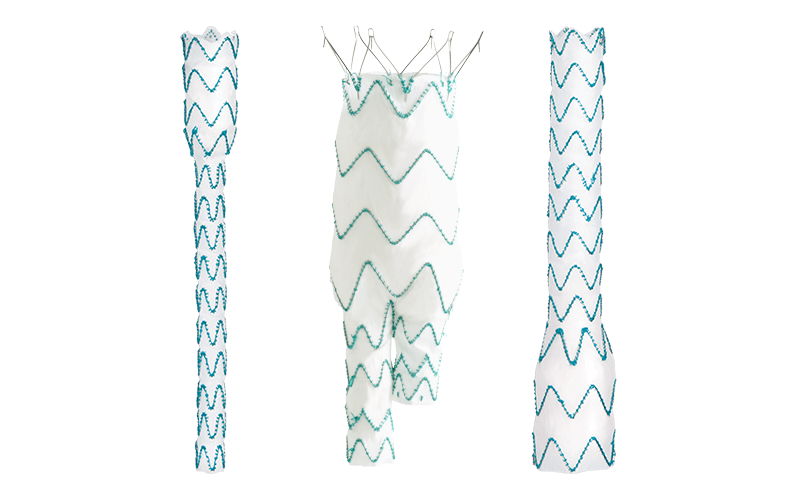
Three-piece system
With a wide range of sizes, diameters and tapers to customise to the needs of each patient.
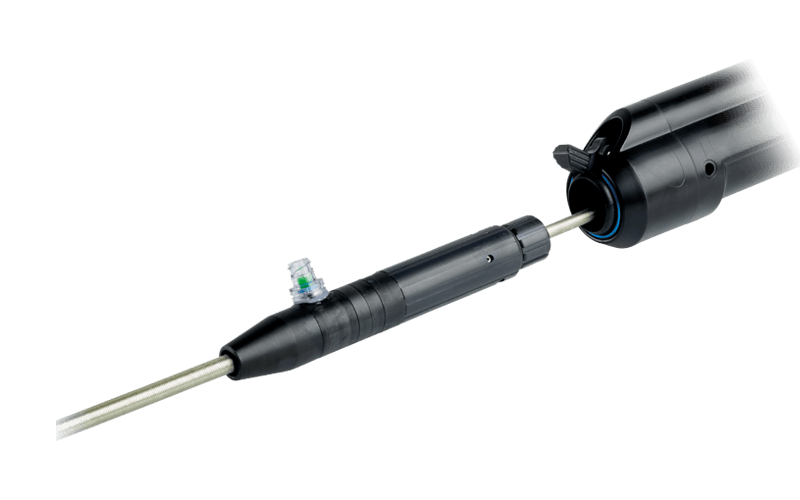
Unique leave-behind sheath
Detaches after delivery to allow for balloon or other access.
Dual fixation for secure sealing

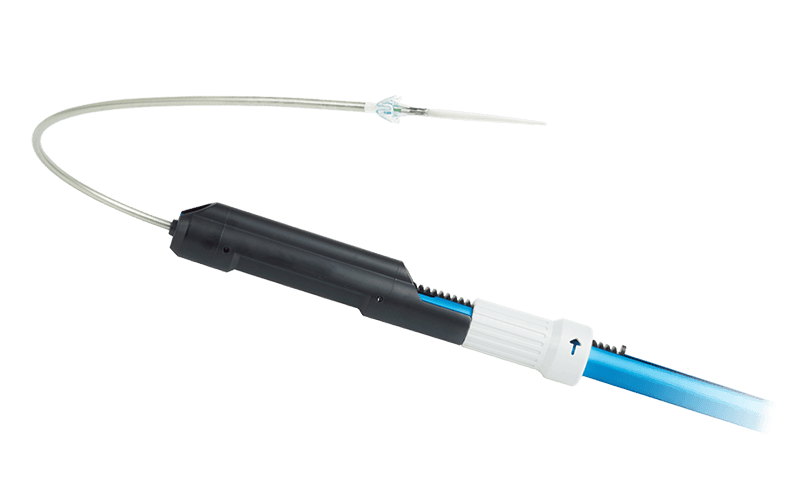
Easy system removal
All components are designed for safe removal, without sharp edges.
Proximal Clasp for Repositioning
Engineered for precise deployment, TREO’s advanced proximal clasp moves distally to deploy


After suffering from an Abdominal Aortic Aneurysm (AAA) Michael’s options were to undergo an open repair or a minimally invasive EVAR procedure using one of our TREO products.
Deployment Animation
Watch the TREO deployment animation
Downloads
Downloads – Features & Benefits
References
Data on file at Terumo Aortic.
Product Disclaimer
Product availability subject to regulatory approval.
An EU Declaration of Conformity may be requested from regulatoryaffairsuk@terumoaortic.com
Instructions for Use
View the eIFU for more information on use, indications, contraindications, warnings/precautions and availability within your market.
Contact us
Click the button below to get in touch. For more updates, follow us on X and LinkedIn. You can also view our VuMedi channel.