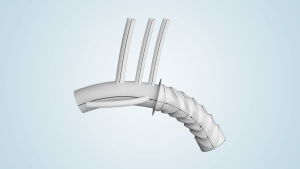
Low profile without compromise
RelayPro offers a low profile system without compromising performance. The platform utilises the same stent design, materials and foundational Dual Sheath Technology of the proven Relay®Plus.
Sources of Reduction*
38% profile reduction
Graft material is a tight woven polyester14% profile reduction
Optimised radiopaque marker positioning24% profile reduction
New thin wall coiled primary introducer outer sheath21% profile reduction
Reduction of inner sheath diameterNo change
Identical stents to RelayPlusNo change
Same suture and sewing method as both RelayPlus and TREO®
*Data on file at Terumo Aortic.
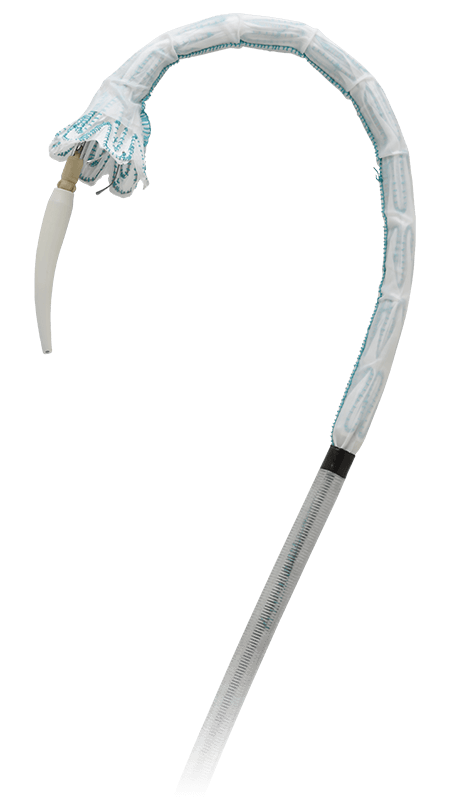
Flexible and stable from navigation to deployment
You can feel confident at each key step in the endovascular procedure with the advanced engineering of the Relay® aortic stent graft system.
Low profile delivery
Optimised weave pattern and radiopaque markers to reduce profile while maintaining stent-graft integrity and visualisation.
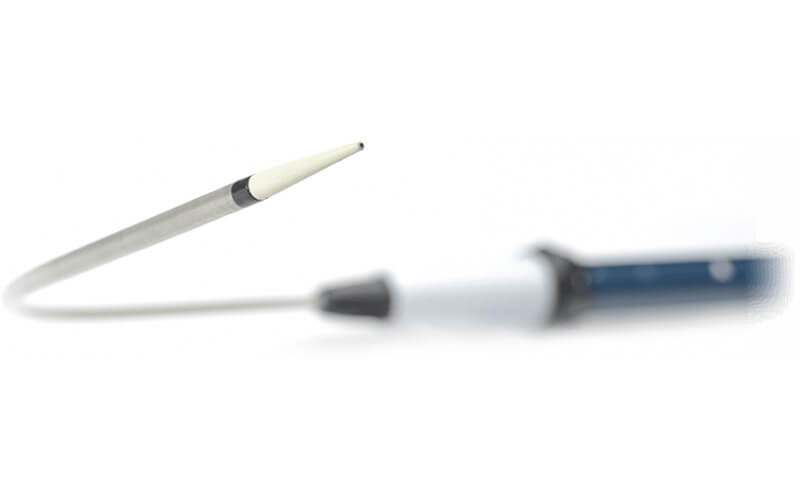

Wide range of bare stent and non bare stent options
A more personalised approach
The RelayPro system has a wide range of sizes and tapers allowing each patient access to the right solution, every time.

Mechanical advantage for enhanced precision
Forward and backward gear system enhances control and delivery of the stent-graft, in both directions.
Optimal Seal
RelayPro NBS uses an engineered Delivery System to deliver perpendicular landing and optimal stent-graft apposition at the inner curve. Optimised weave pattern and radiopaque markers to reduce profile while maintaining stent-graft integrity and visualisation.

Deployment Animation
Watch the RelayPro deployment animation
References
Data on file at Terumo Aortic.
Downloads
Product Disclaimer
Product availability subject to regulatory approval.
An EU Declaration of Conformity may be requested from regulatoryaffairsuk@terumoaortic.com
Instructions for Use
View the eIFU for more information on use, indications, contraindications, warnings/precautions and availability within your market.
Contact a representative
Discuss your patient’s aortic repair or learn more about our solutions for every segment of the aorta.