Atrium Opening Signals Ambition
The opening ceremony, performed by CEO Erik Pomp and Brian Coll, the longest serving member of the Terumo Aortic family with over 37 years’ service, marks a major milestone in our investment project which will see over £50 million spent to expand and develop the manufacturing site. Erik Pomp, CEO of Terumo Aortic, commented: “We […]
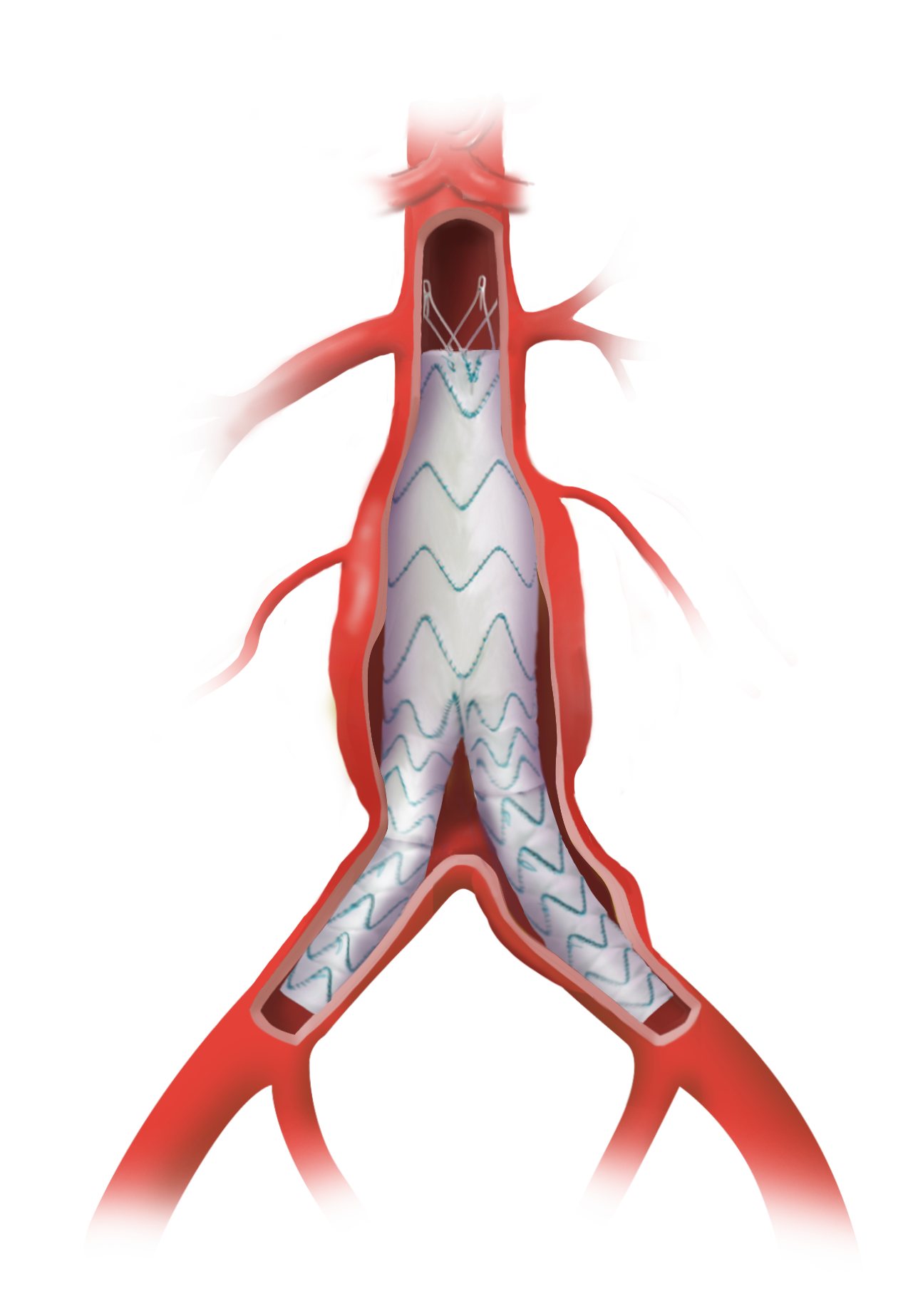
Terumo Aortic announces enrolment of patients in an all-comers TREO AAA study
The TREO Abdominal Stent-Graft System is approved by the US Food and Drug Administration (FDA) to treat patients with abdominal aortic aneurysms. This is a prospective, multi-center, non-randomised, single-arm, post-market study; the purpose is to evaluate the real world, long-term performance of the device as a treatment for patients with infrarenal abdominal aortic aneurysms or […]
Terumo Aortic announces US FDA approval for Thoraflex Hybrid
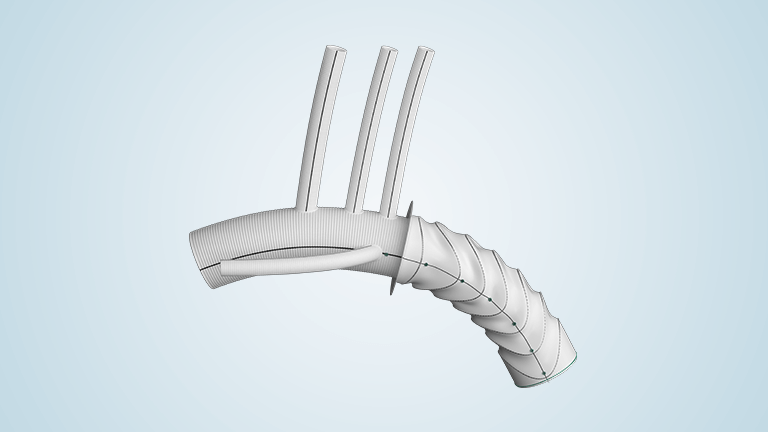
Thoraflex Hybrid is the first of its kind device used in Frozen Elephant Trunk (FET) repair in the United States and was granted Breakthrough Device Designation by the FDA in 2020. Thoraflex Hybrid is a single use medical device combining a Gelweave polyester graft with a Nitinol self-expanding stent graft and is indicated for the […]
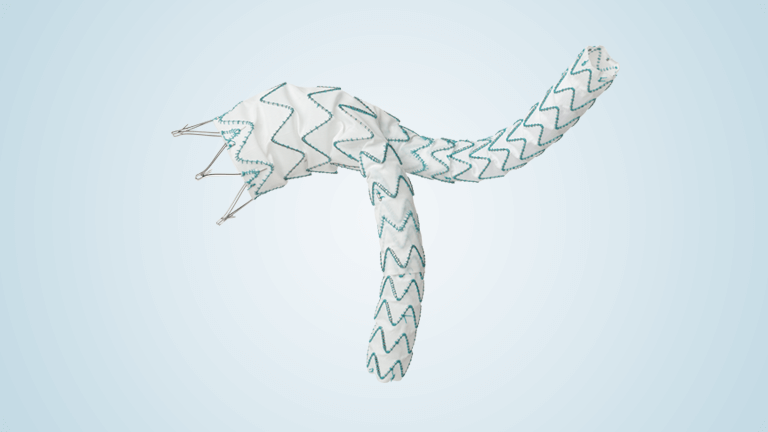
Terumo Aortic announces US FDA breakthrough device designation for RelayBranch
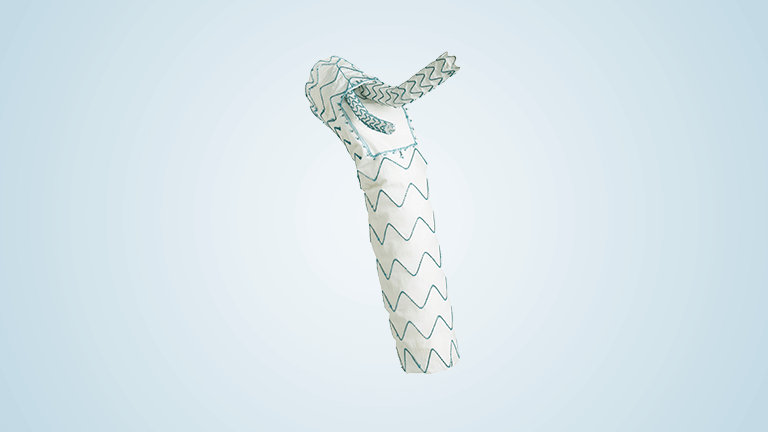
The RelayBranch Thoracic Stent-Graft System is implanted in patients with thoracic aortic arch pathologies requiring treatment that includes coverage of the innominate and left common carotid arteries. The purpose of the FDA’s Breakthrough Device Designation program is to fast-track the regulatory review process for certain medical technologies and device-led combination products that satisfy certain criteria; […]
Terumo Aortic announces launch and first commercial use of the Aortic Balloon device
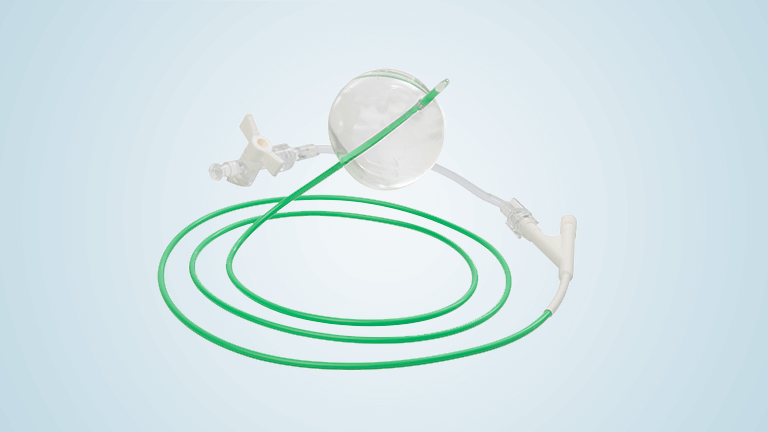
The device assists physicians in the expansion of the aorta when using TREO and RELAY stent-grafts in endovascular aortic repair. Complementing our market leading product range of surgical and endovascular grafts, the Aortic Balloon is a low profile device with 10 F and offers exceptional inflation control and the broadest balloon diameter range, up to […]
Terumo Aortic announces PMDA Approval and first commercial implant of TREO endovascular device in Japan

This first implant was undertaken by Takao Ohki, MD, Chairman of the Department of Surgery, Professor and Chief of the Division of Vascular Surgery, Jikei University School of Medicine, Tokyo. Professor Ohki commented: “The procedure was very successful, the device performed well, and the patient is making a good recovery. The major concerns in endovascular […]
Terumo Aortic announces publication of the primary endpoint results from the TREO Pivotal Study

Following recent approval by the US Food and Drug Administration (FDA) of the TREO® Abdominal Aortic Stent-Graft System for the treatment of patients with abdominal aortic aneurysms (AAA), Terumo Aortic announced today the publication of the primary endpoint results from the investigational device exemption (IDE) pivotal study in the Journal of Vascular Surgery.
Terumo Creates Global Group Music

The composition is based on the concept “Let’s respect the past, dream for the future, and open new doors together.” The track is composed by Mr. Akira Senju, a highly regarded Japanese composer and recipient of numerous awards and honors. Mr. Senju’s 35th anniversary album which was released on October 21 includes ‘TOGETHER’, one of […]
Terumo Aortic announces US FDA Approval for TREO endovascular device

TREO is the only device commercially available for endovascular aneurysm repair (EVAR) with dual proximal fixation and lock stent technology offering physicians the next advancement in endovascular device solutions. It is a three-piece design featuring in situ limb adjustability and provides a wide range of aortic device configurations to specifically address the anatomy of each […]
Terumo Aortic Announces US FDA Breakthrough Device designation for Thoraflex Hybrid device

The purpose of the FDA’s Breakthrough Device Designation program is to fast-track the regulatory review process for certain medical technologies and device-led combination products that satisfy certain criteria; these include providing a more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions. The aim of the program is to provide patients and […]