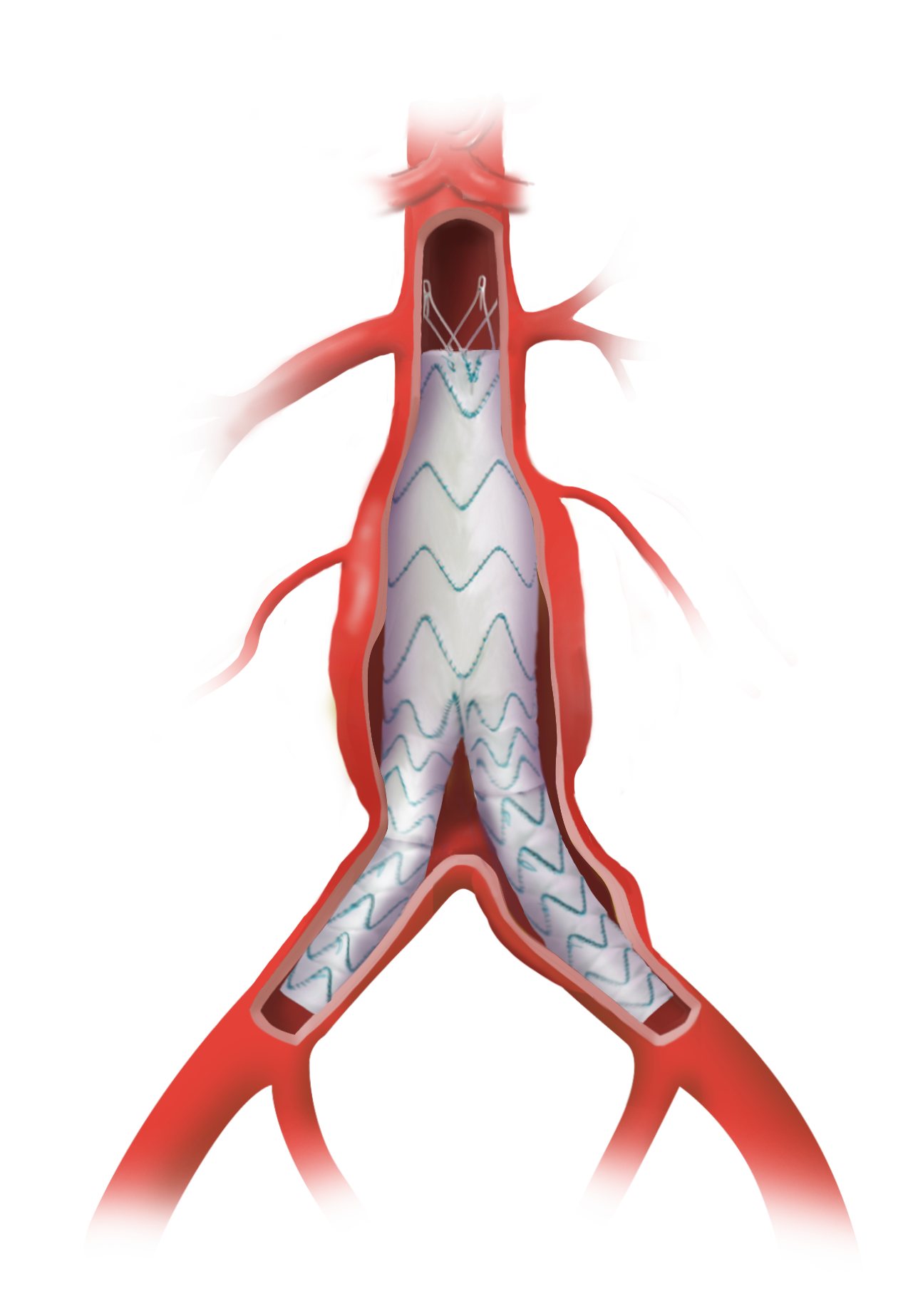
Thoraflex Hybrid is the first of its kind device used in Frozen Elephant Trunk (FET) repair in the United States and was granted Breakthrough Device Designation by the FDA in 2020.
Thoraflex Hybrid is a single use medical device combining a Gelweave polyester graft with a Nitinol self-expanding stent graft and is indicated for the open surgical repair or replacement of damaged or diseased vessels of the aortic arch and descending aorta with or without involvement of the ascending aorta in cases of aneurysm and/or dissection.
Joseph Coselli, MD (Professor, Executive Vice Chair, Division of Cardiothoracic Surgery, Baylor College of Medicine, Houston, Texas, US) the Principal Investigator commented: “This approval represents a significant milestone in the treatment of patients who need total aortic arch replacement and have significant disease of the descending thoracic aorta. They can now be treated anytime in a single stage procedure with this hybrid device rather than two procedures which has been the conventional pathway in the United States for this group of patients. This, in turn, has led to lowering the risk of major adverse events by 22.6%, in the first year, over traditional treatments.”
Dr. Coselli continued: “Thoraflex Hybrid facilitates secondary interventions for distal extension and, in the United States, is designated for usage with Terumo Aortic’s RelayPro NBS device. This unique labelling aspect provides surgeons with additional confidence should patients have continued aortic disease progression.”
Paul Kuznik, President of Terumo Aortic North America, added: “The FDA approval of Thoraflex Hybrid is a tremendous opportunity for Terumo Aortic in the United States. This innovative hybrid device complements our open surgical graft and endovascular portfolio making us one of the strongest medical device companies within the aortic space, helping to deliver our commitment to provide solutions for every aorta.”
Thoraflex Hybrid received CE Mark approval in 2012 with more than 13,000 devices sold commercially around the world over the past 10 years. This device is integral to Terumo Aortic’s market-leading portfolio of surgical, endovascular and hybrid devices to treat every segment of the aorta.