Terumo Aortic announces FDA approval of dissection and transection indication expansion for the Relay®Pro Stent-Graft system in the United States
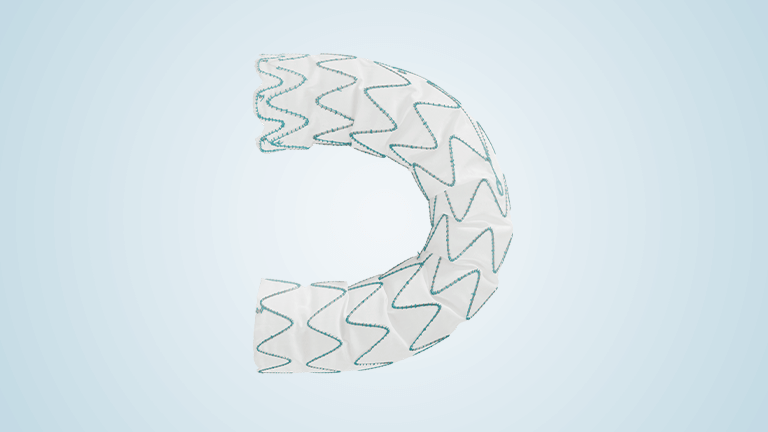
Relay®Pro delivers uncompromised clinical performance in the lowest commercially available profile and is indicated for all pathologies of the descending thoracic artery; aneurysm, penetrating atherosclerotic ulcers, transection and dissection. Relay®Pro offers unparalleled accuracy, proven efficacy and durability as well as the widest range of sizes, tapering and configurations on the market, designed to expand the […]
Terumo Aortic announces first implant of innovative custom-made thoracoabdominal hybrid device in North America
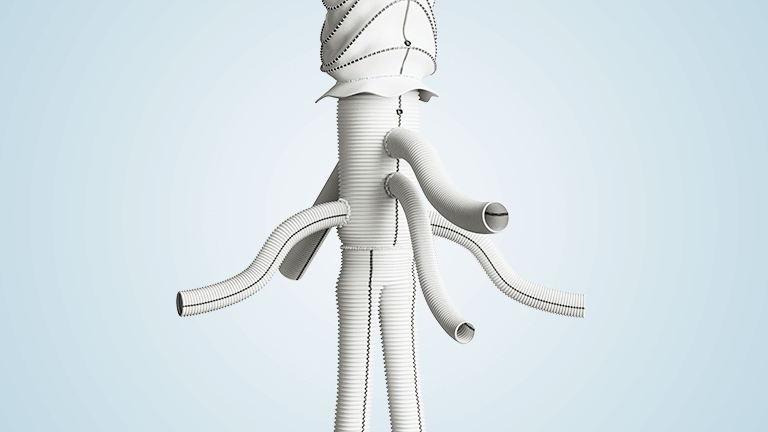
This thoracoabdominal repair procedure using Thoracoflo™ was carried out by Dr Randy Moore, Co-Director of the Calgary Complex Aortic Program at the University of Calgary in Canada. Dr Moore and the team at University of Calgary were supported by Professor Sabine Wipper, Chair of Vascular Surgery, University Hospital Innsbruck, Austria, who continues to be a […]
Terumo Aortic announces new technology add-on payment for Thoraflex Hybrid device in the United States
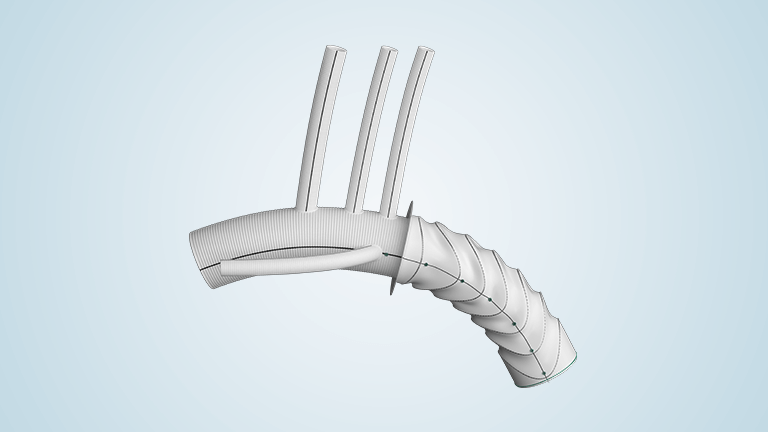
Thoraflex Hybrid is the first of its kind device used in Frozen Elephant Trunk (FET) repair in the United States. It was granted Breakthrough Device Designation by the Food and Drug Administration (FDA) in 2020 followed by FDA approval for commercial sale in the United States earlier this year. This innovative hybrid device allows patients […]
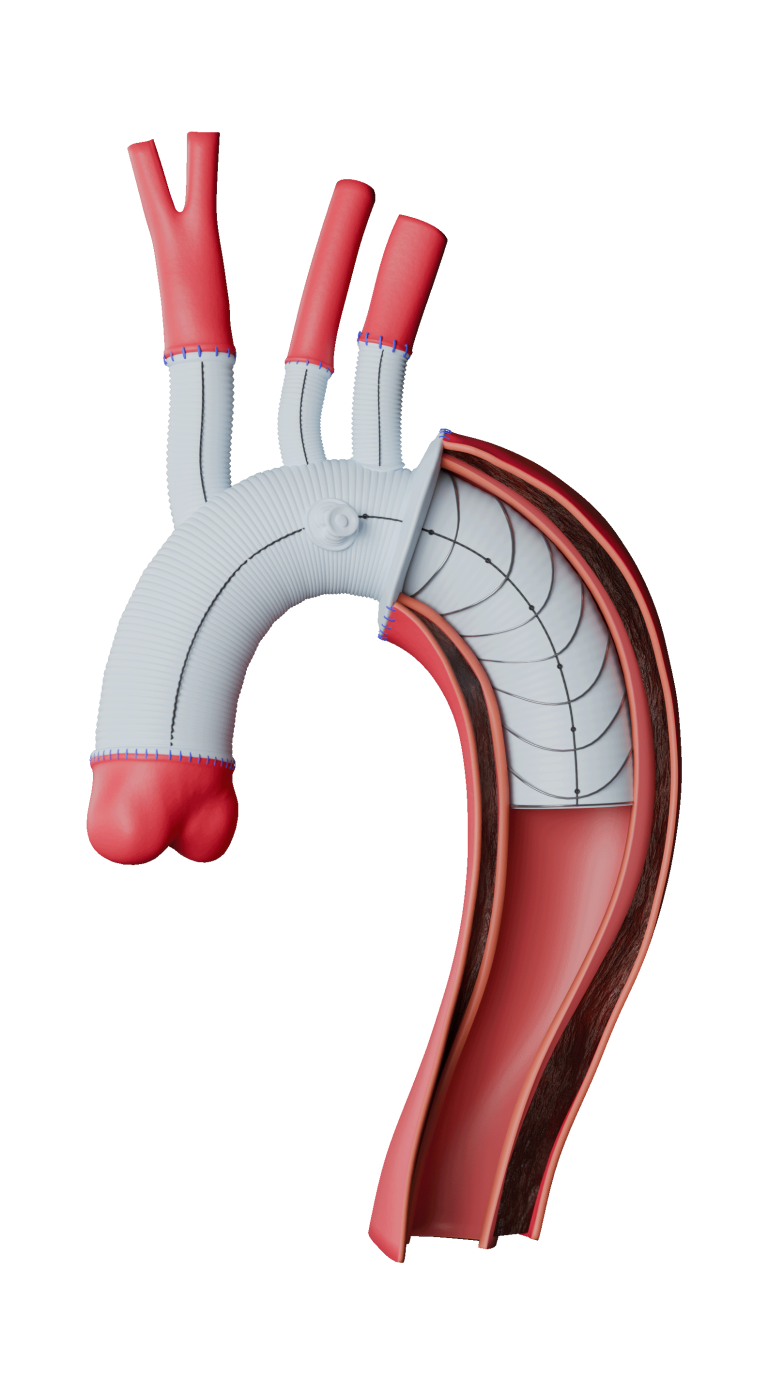
Terumo Aortic announces first commercial implant of the Thoraflex Hybrid device in the USA

Thoraflex Hybrid is a single use medical device combining a Gelweave polyester graft with a Nitinol self-expanding stent graft. It is indicated for the open surgical repair or replacement of damaged or diseased vessels of the aortic arch and repair of the descending thoracic aorta with or without involvement of the ascending aorta, in cases […]
Atrium Opening Signals Ambition
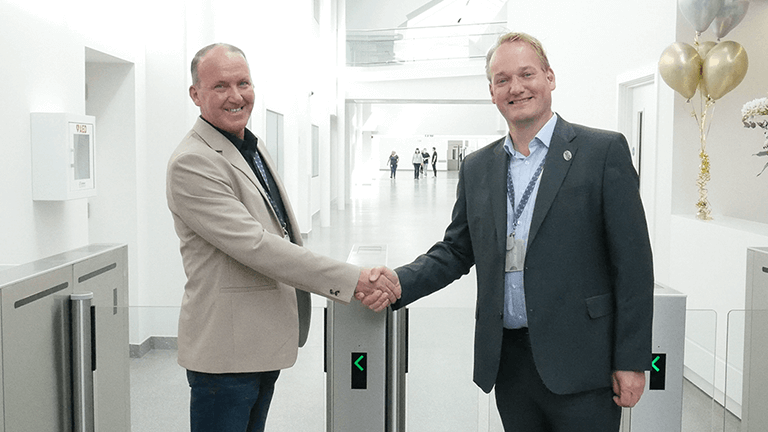
The opening ceremony, performed by CEO Erik Pomp and Brian Coll, the longest serving member of the Terumo Aortic family with over 37 years’ service, marks a major milestone in our investment project which will see over £50 million spent to expand and develop the manufacturing site. Erik Pomp, CEO of Terumo Aortic, commented: “We […]
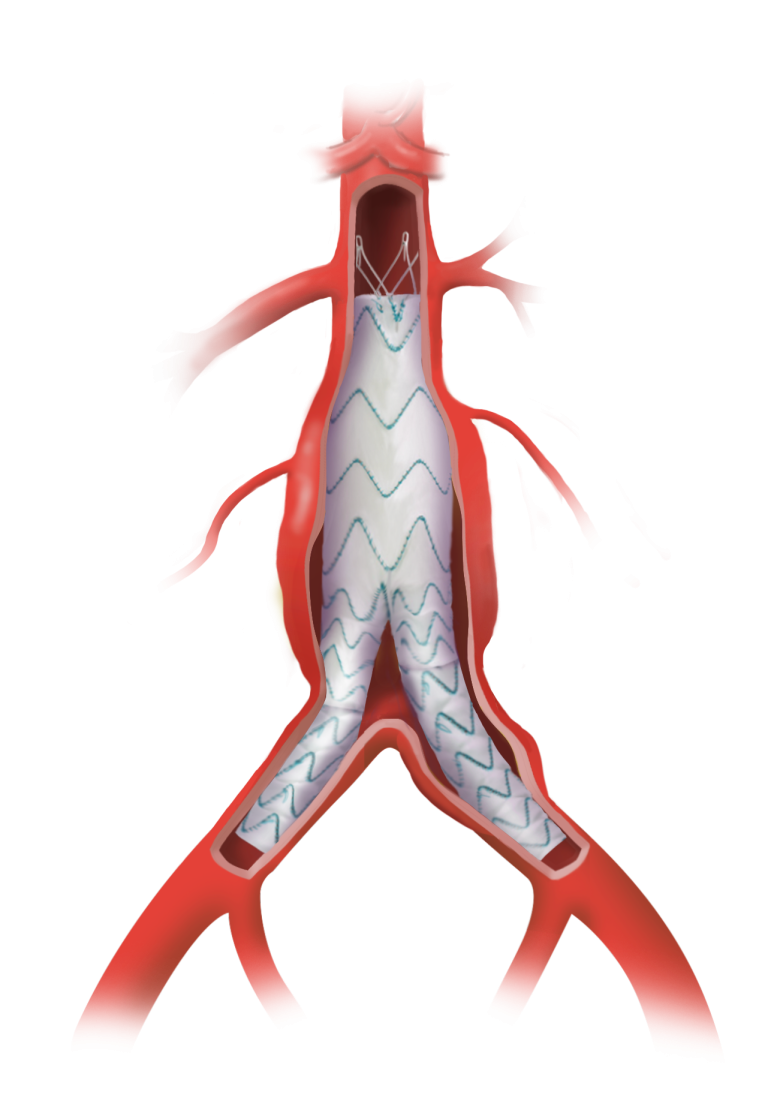
Terumo Aortic announces enrolment of patients in an all-comers TREO AAA study
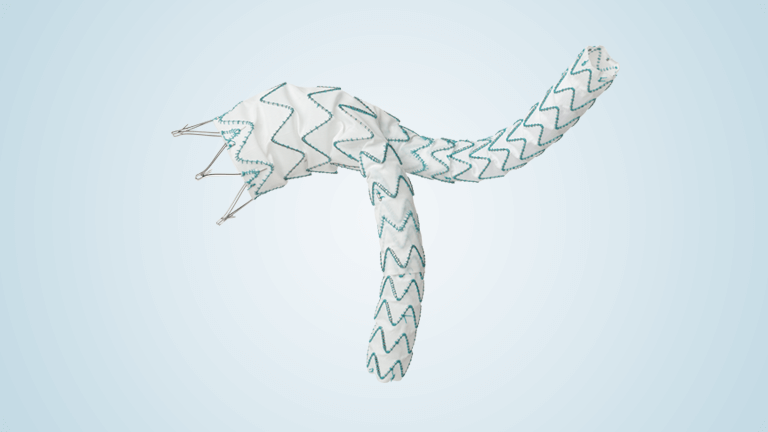
The TREO Abdominal Stent-Graft System is approved by the US Food and Drug Administration (FDA) to treat patients with abdominal aortic aneurysms. This is a prospective, multi-center, non-randomised, single-arm, post-market study; the purpose is to evaluate the real world, long-term performance of the device as a treatment for patients with infrarenal abdominal aortic aneurysms or […]
Terumo Aortic announces US FDA approval for Thoraflex Hybrid

Thoraflex Hybrid is the first of its kind device used in Frozen Elephant Trunk (FET) repair in the United States and was granted Breakthrough Device Designation by the FDA in 2020. Thoraflex Hybrid is a single use medical device combining a Gelweave polyester graft with a Nitinol self-expanding stent graft and is indicated for the […]
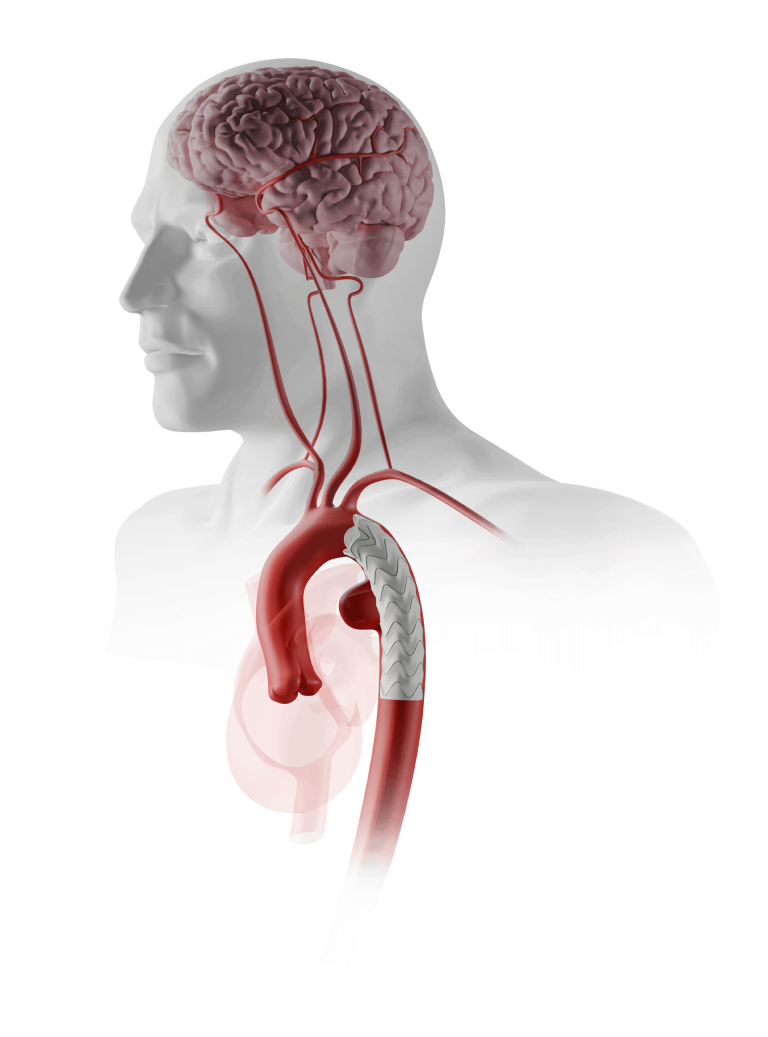
Terumo Aortic announces US FDA breakthrough device designation for RelayBranch
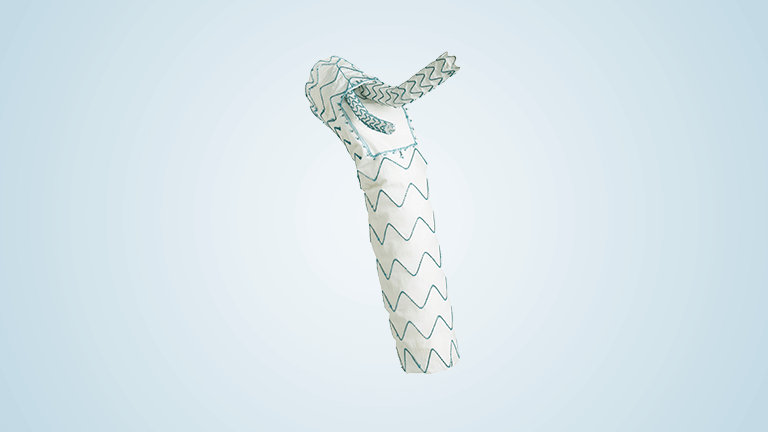
The RelayBranch Thoracic Stent-Graft System is implanted in patients with thoracic aortic arch pathologies requiring treatment that includes coverage of the innominate and left common carotid arteries. The purpose of the FDA’s Breakthrough Device Designation program is to fast-track the regulatory review process for certain medical technologies and device-led combination products that satisfy certain criteria; […]
Terumo Aortic announces launch and first commercial use of the Aortic Balloon device
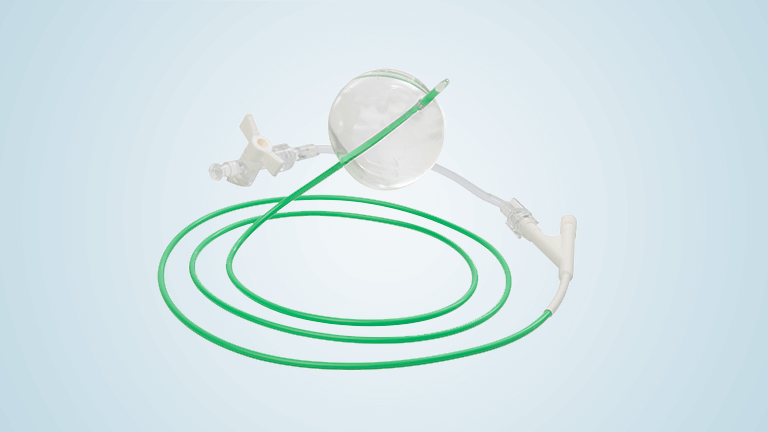
The device assists physicians in the expansion of the aorta when using TREO and RELAY stent-grafts in endovascular aortic repair. Complementing our market leading product range of surgical and endovascular grafts, the Aortic Balloon is a low profile device with 10 F and offers exceptional inflation control and the broadest balloon diameter range, up to […]
Terumo Aortic announces PMDA Approval and first commercial implant of TREO endovascular device in Japan

This first implant was undertaken by Takao Ohki, MD, Chairman of the Department of Surgery, Professor and Chief of the Division of Vascular Surgery, Jikei University School of Medicine, Tokyo. Professor Ohki commented: “The procedure was very successful, the device performed well, and the patient is making a good recovery. The major concerns in endovascular […]