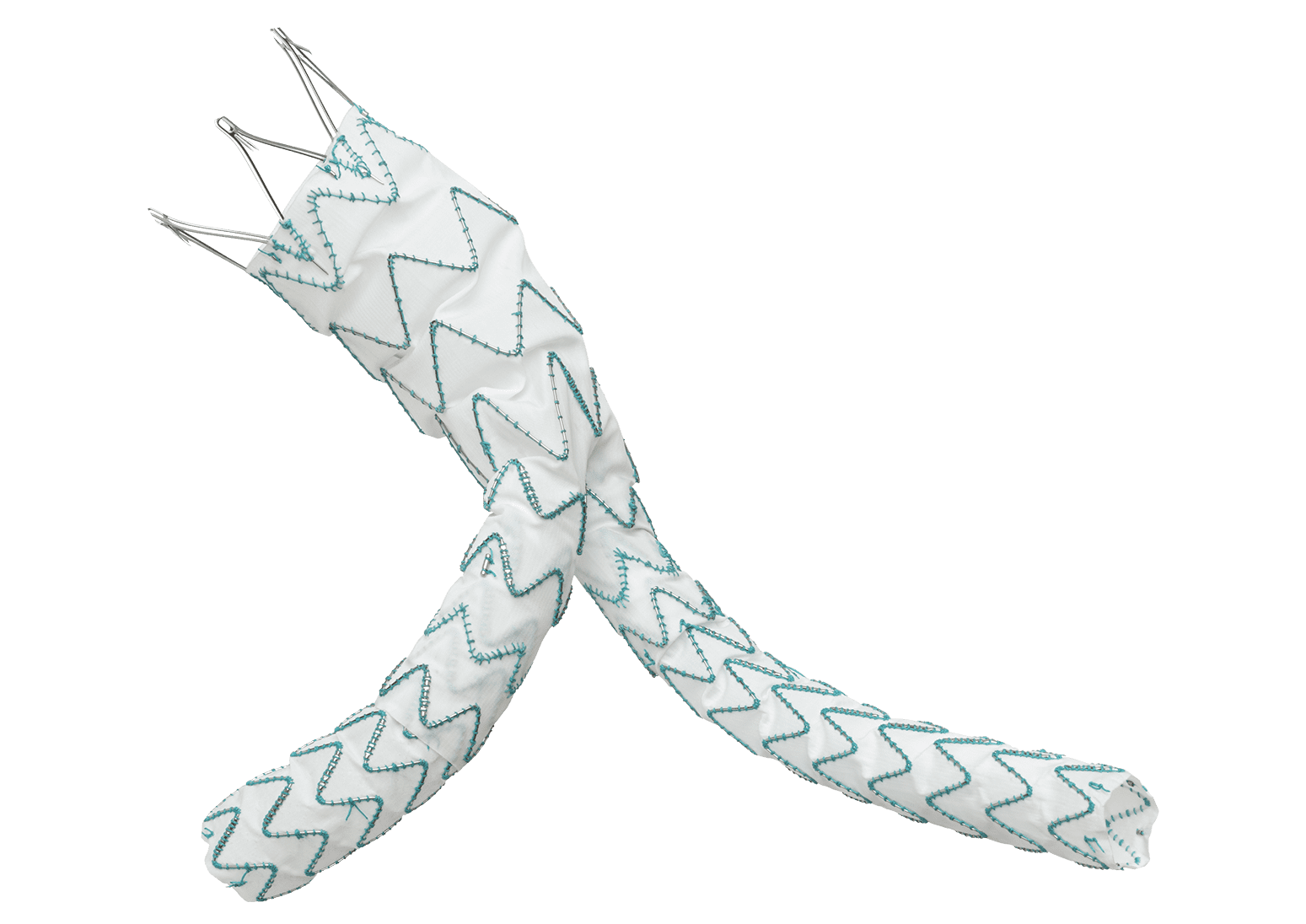
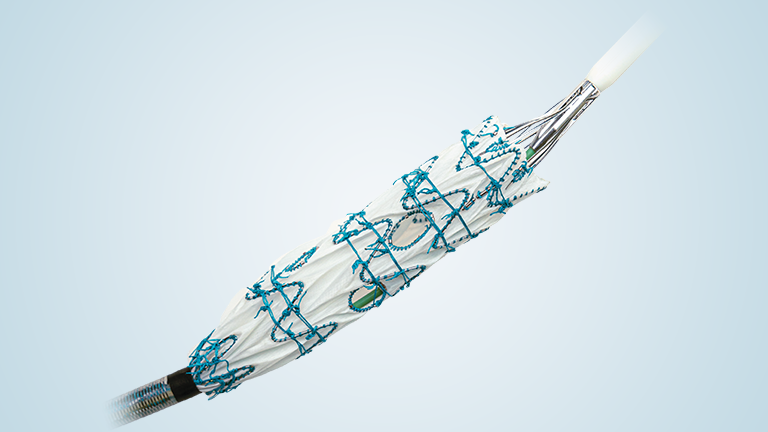
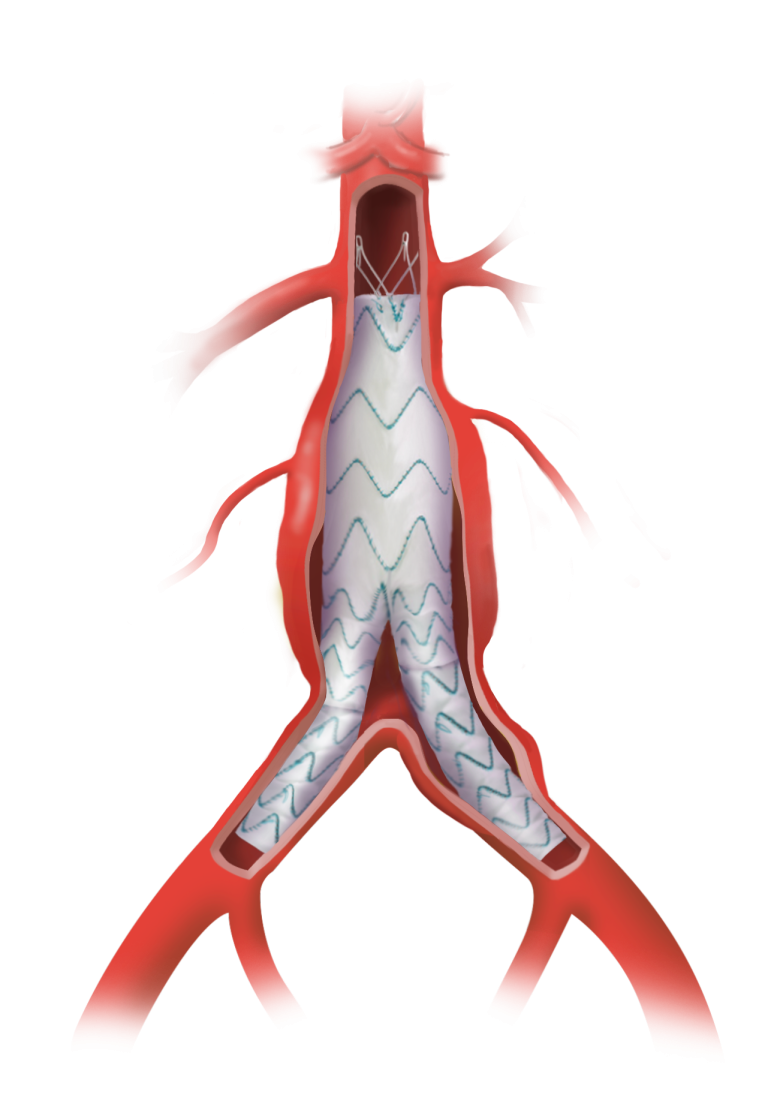
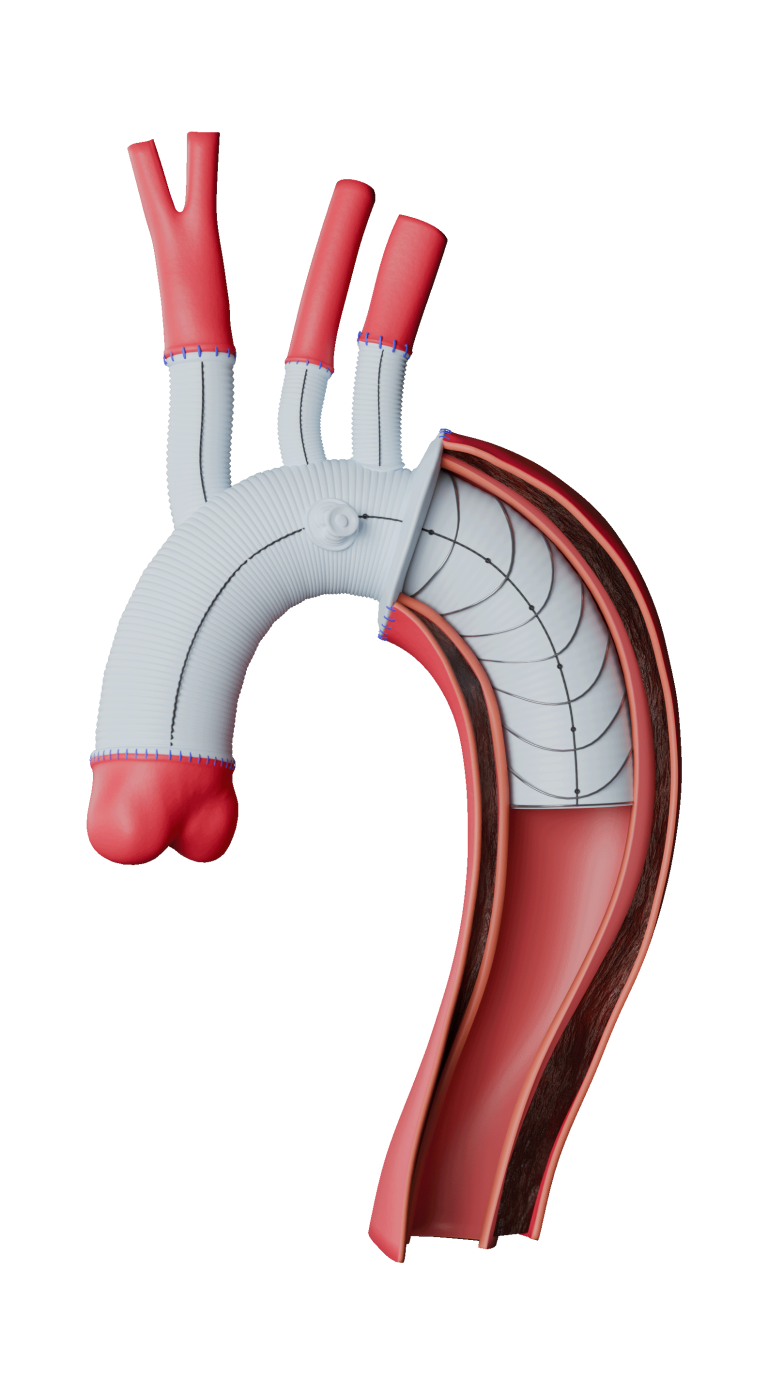
TREO is the only device commercially available for endovascular aneurysm repair (EVAR) with dual proximal fixation and lock stent technology offering physicians the next advancement in endovascular device solutions. It is a three-piece design featuring in situ limb adjustability and provides a wide range of aortic device configurations to specifically address the anatomy of each individual patient. TREO is designed for accurate, controlled and safe deployment; the exclusive proximal clasping mechanism and a unique leave-behind sheath simplify the procedure as highlighted in recent clinical studies.
Scott Rush, Terumo Aortic’s Vice President of Research and Development said, “We set out to provide a AAA system with the most comprehensive set of benefits available and believe that we have an implant and delivery system that is unmatched in terms of design features. The tri-modular design and in situ sizing of TREO allow physicians to deliver a tailored solution by selecting components precisely sized and joined for each patient’s anatomy.”
“Stent migration and endoleaks are major concerns in EVAR,” said Matt Eagleton, MD (Boston, MA), the National Principal Investigator of the IDE Study. “The TREO stent-graft offers both suprarenal and infrarenal fixation, distributing the stent-graft fixation in two different anatomical levels which may work to reduce potential migration. I have been very impressed with the results of the trial, particularly in the sac shrinkage data. I expect this to lower re-intervention rates in my practice.”
Paul Kuznik, President of Terumo Aortic North America, commented, “This approval represents a tremendous opportunity for Terumo Aortic in the United States. We recently complemented our sales organisation with our open surgical graft portfolio and now, with the addition of TREO, we are shaping up to be one of the strongest companies within the aortic space fulfilling our commitment to find solutions for every aorta.”
TREO received CE Mark approval in 2015 and is integral to Terumo Aortic’s market-leading portfolio of surgical, endovascular and hybrid devices to treat every segment of the aorta.