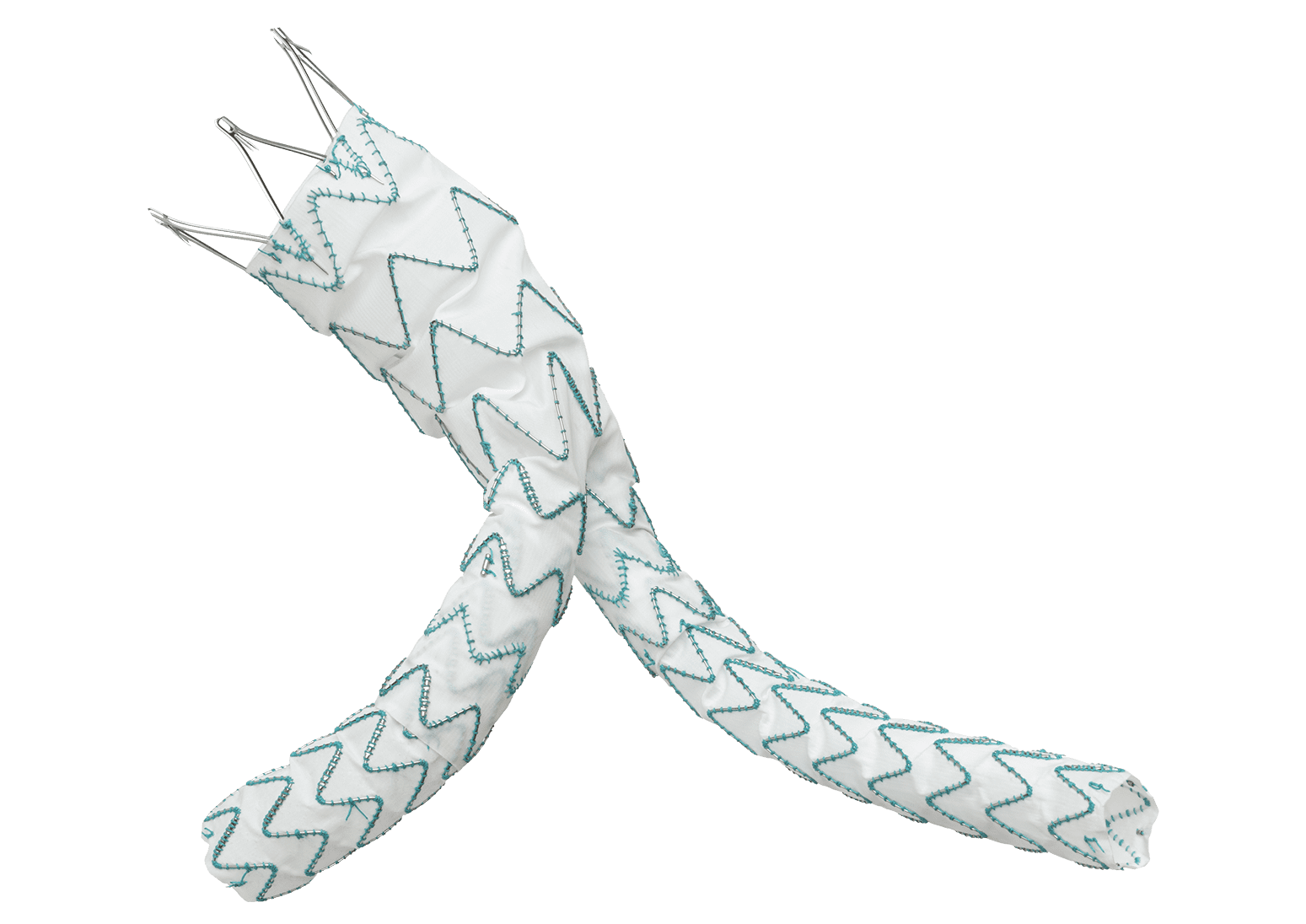
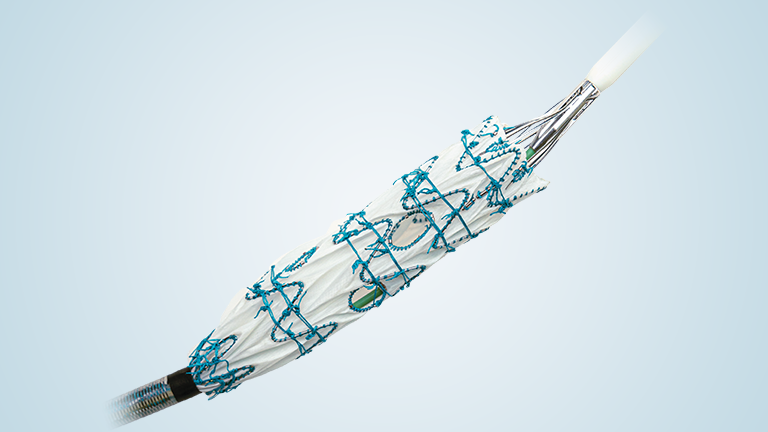
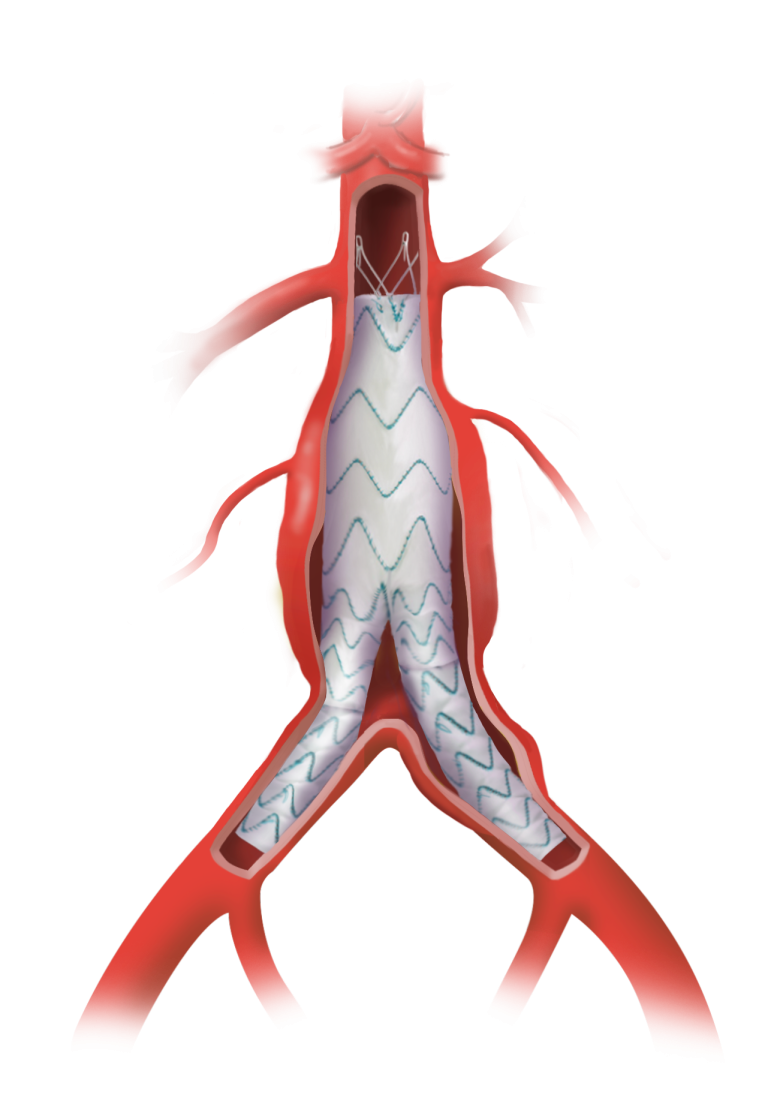
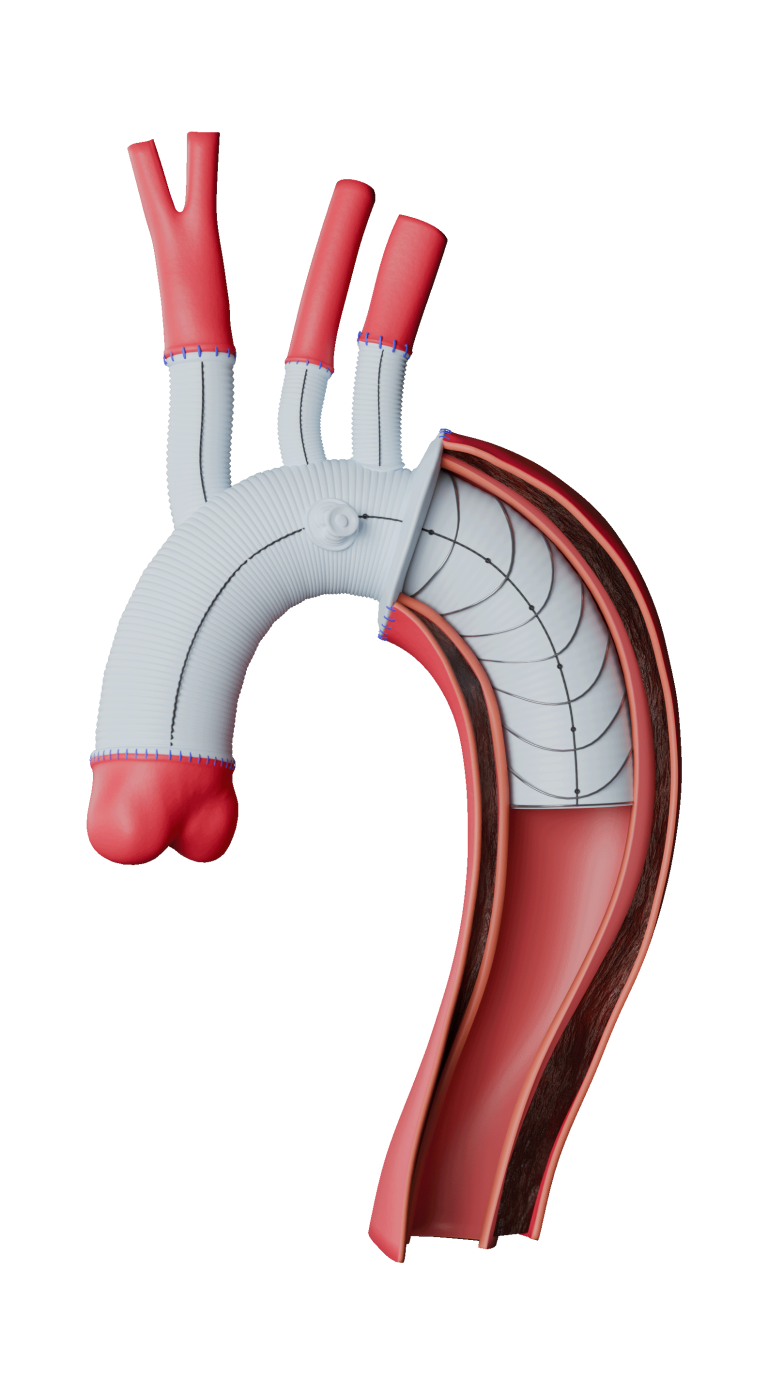
Following recent approval by the US Food and Drug Administration (FDA) of the TREO® Abdominal Aortic Stent-Graft System for the treatment of patients with abdominal aortic aneurysms (AAA), Terumo Aortic announced today the publication of the primary endpoint results from the investigational device exemption (IDE) pivotal study in the Journal of Vascular Surgery.
Over a three-year period, a multicentre, non-randomised, investigational device exemption (IDE) pivotal study assessed the safety and effectiveness of TREO®. A total of 150 patients with infrarenal abdominal aortic or aorto-iliac aneurysms were treated in 29 centres across the United States. Follow-up continues but the primary safety and effectiveness endpoint analysis is now complete and demonstrated 100% technical success, only 0.7% incidence of major adverse events at 30 days, and 93.1% successful aneurysm treatment at one year.
In line with previous studies such as the global RATIONALE registry, a high percentage of patients had positive aortic remodeling with 54.3% who had aneurysm sac shrinkage > 5 mm at three years.
Principal investigator of the study and author, Matthew J. Eagleton, MD (Chief, Division of Vascular and Endovascular Surgery and Co-Director, Fireman Vascular Center, Massachusetts General Hospital, Boston, US) commented: “TREO® is a new generation EVAR device designed to improve deployment and fixation, and increase the potential to treat more complex aortas with a wider range of sizing options and a number of engineering features to ensure durability for better longer-term outcomes. Research continues with ongoing follow-up of the IDE cohort for a maximum of 10 years.”
One of the authors of the study, Michael C. Stoner, MD (Professor and Chief, Vascular Surgery, University of Rochester, New York, US) commented on the consistently good sac regression observed with TREO® saying: “Worldwide experience with this device along with publications from our groups have already suggested that TREO is linked to positive remodeling. Certainly, data to three years in the IDE study are in line with those earlier findings and, as we gain more experience with TREO, it will be beneficial to develop an even better understanding of the physiological factors contributing to sac regression.”