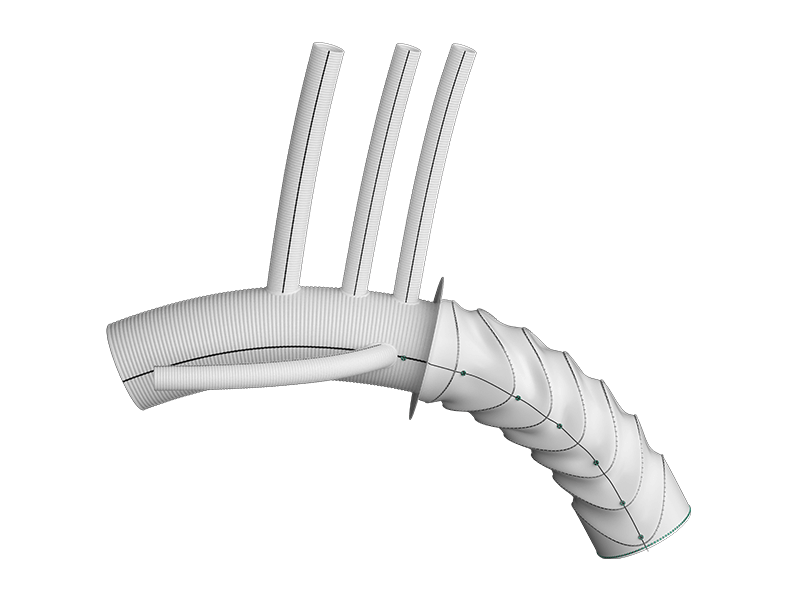
Thoraflex Hybrid is the first of its kind device used in Frozen Elephant Trunk (FET) repair in the United States. It was granted Breakthrough Device Designation by the Food and Drug Administration (FDA) in 2020 followed by FDA approval for commercial sale in the United States earlier this year. This innovative hybrid device allows patients with suitably limited disease to be treated in a single stage procedure rather than two procedures which has previously been the conventional pathway in the United States for this group of patients.
The add-on payment will allow hospitals to be reimbursed for the incremental costs relating to the implantation of the Thoraflex Hybrid Frozen Elephant Trunk (FET) device to support the treatment of patients with complex aortic arch disease – this is in addition to the Diagnosis Related Group (DRG) reimbursement.
The add-on payment is effective in CMS’ FY23 fiscal year, starting on 1 October 2022 and has been assigned the maximum new technology add-on payment – 65% of the average cost of the technology, for a case involving the use of the Thoraflex Hybrid Device.
Jeffrey Mifek, Global Vice President of Clinical and Medical Affairs for Terumo Aortic commented: “We are delighted to have received this approval from CMS and it represents an important milestone for Terumo Aortic. The add-on payment provides further evidence that the Thoraflex Hybrid device significantly improves overall patient outcomes, compared to other available treatments.”
Thoraflex Hybrid received CE Mark approval in 2012 with more than 13,000 devices sold commercially around the world over the past 10 years. This device is integral to Terumo Aortic’s market-leading portfolio of surgical, endovascular and hybrid devices to treat every segment of the aorta.