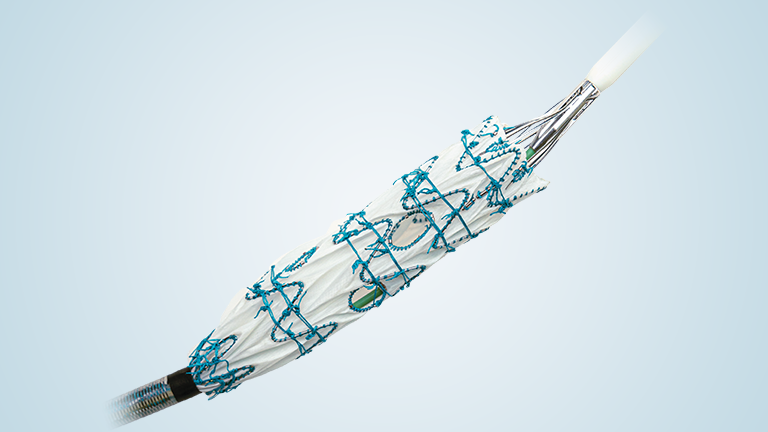
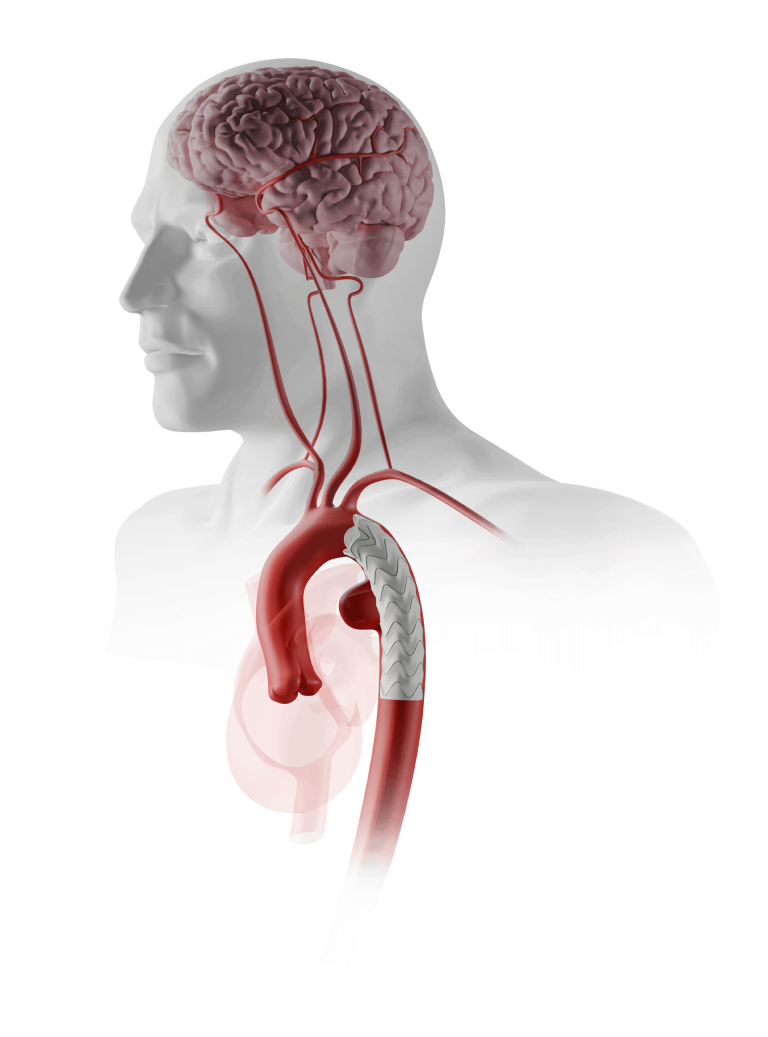
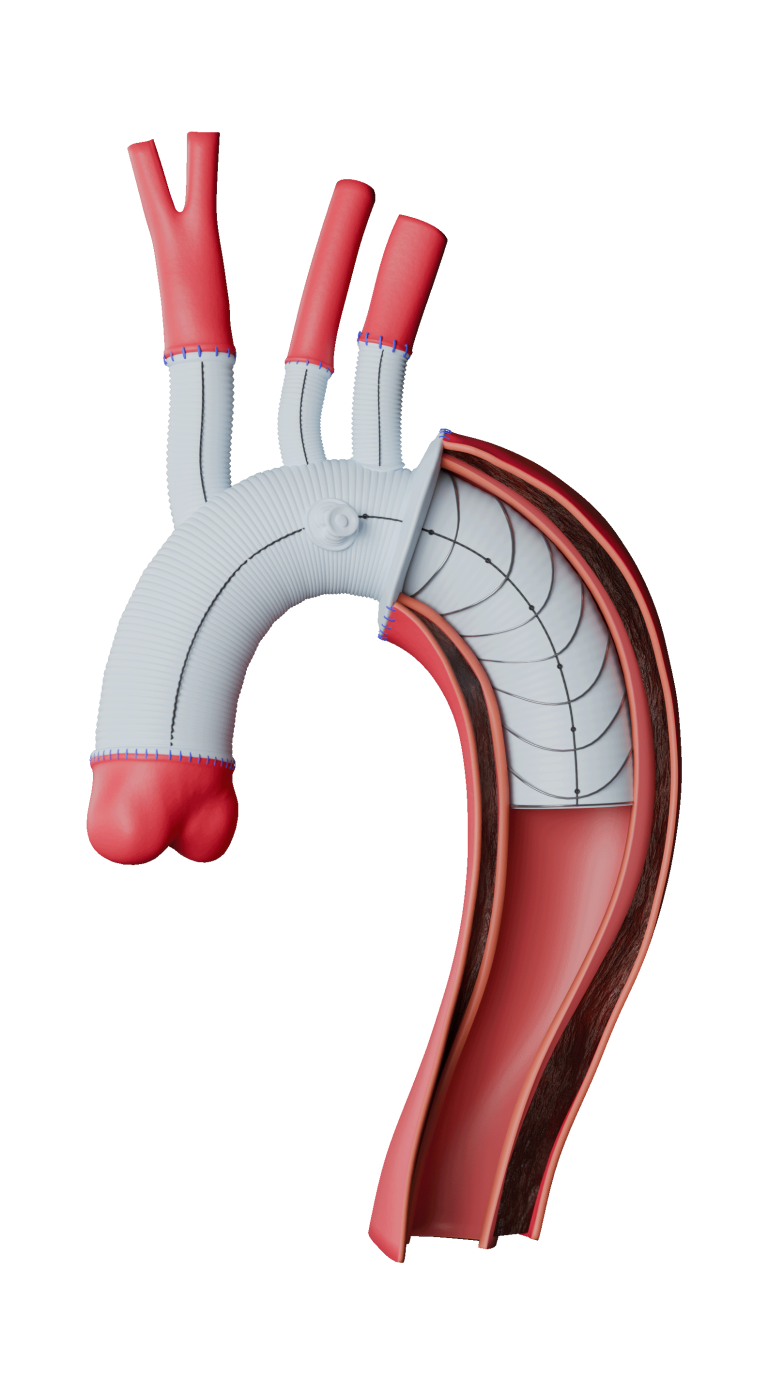
Terumo Aortic today announced enrolment of the first patient in the RapidLink™ pivotal Investigational Device Exemption (IDE) study in the US and Europe*. The study is designed to evaluate the safety and effectiveness of the RapidLink™ device for the repair or replacement of supra-aortic vessels during open surgical repair of aortic disease involving the thoracic aorta.
The study will collect information on patients who are already having surgery to repair their aorta and who will have the RapidLink™ device implanted into one or more of the aortic arch vessels.
Achieving the first enrolment in this FDA approved IDE is a major milestone that introduces an intuitive deployment system that integrates seamlessly into existing surgical workflows. Its design reduces the number of procedural steps, enabling faster and more precise graft placement. By streamlining the hybrid approach, RapidLink™ will allow more surgeons to treat complex aortic arch pathologies with confidence and consistency.
The study will include up to 30 centres in the US and Europe. We anticipate up to 150 patients will be enrolled across the two study arms: elective and emergent. The device is designed to be used in all three supra-aortic vessels for repair or replacement. Patients will then be followed for a minimum of 2 years post procedure.
The first patient was enrolled by Puja Kachroo, MD, Associate Professor of Cardiac Surgery and Co-Director of the Aortic Center at Washington University in St. Louis.
The Global Principal Investigator, Malakh Shrestha, MBBS, FRCS (Eng), PhD, Director of Mayo Clinic Aortic Center, Director of Aortic Surgery, Cardio Vascular Surgery, Professor of Surgery, Mayo College of Medicine & Science The Mayo Clinic commented, “The RapidLink™ device provides a transformative solution for complex aortic arch procedures. It simplifies the most technically demanding aspects of hybrid aortic arch repair, reducing procedural complexity, operative time, and patient risk.”
Jeffrey Mifek, Global Vice President, Clinical and Medical Affairs at Terumo Aortic said: “Today we are delighted to mark the first implant of the RapidLink™ device in this Pivotal Study. RapidLink™ has the potential to revolutionise aortic arch surgery. Its ability to make these advanced surgeries available to more patients will greatly improve patient care and further demonstrates Terumo Aortic’s commitment to advancing aortic care.”
*CAUTION — INVESTIGATIONAL DEVICE. Limited by Federal law to investigational use