Terumo Aortic announces launch of the global Thoraflex Hybrid extend study
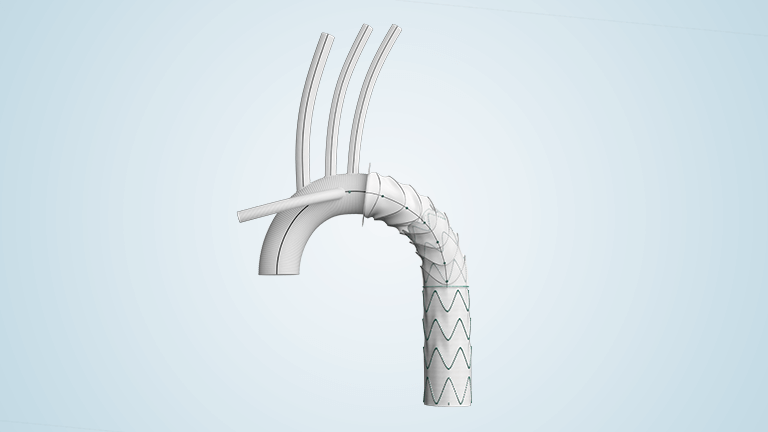
Terumo Aortic today announced the launch of the company’s global Post-Approval Study (PAS) known as EXTEND for Thoraflex Hybrid, the only Frozen Elephant Trunk (FET) device approved by the US Food and Drug Administration (FDA) for the treatment of patients with complex aortic arch disease. This study is a prospective, multi-center, non-randomized, single arm, all-comers, […]
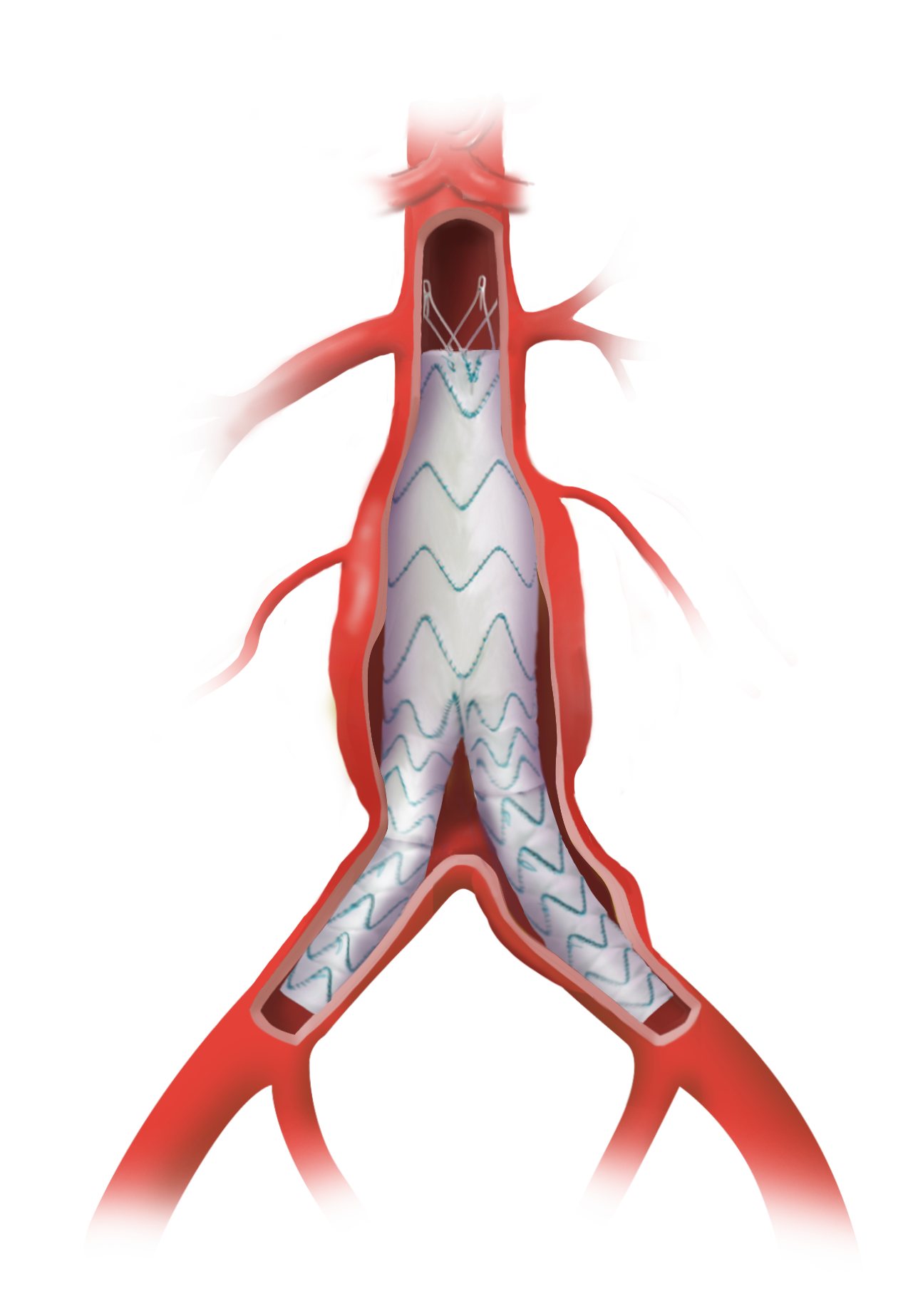
Terumo Aortic announces enrolment of patients in an all-comers TREO AAA study
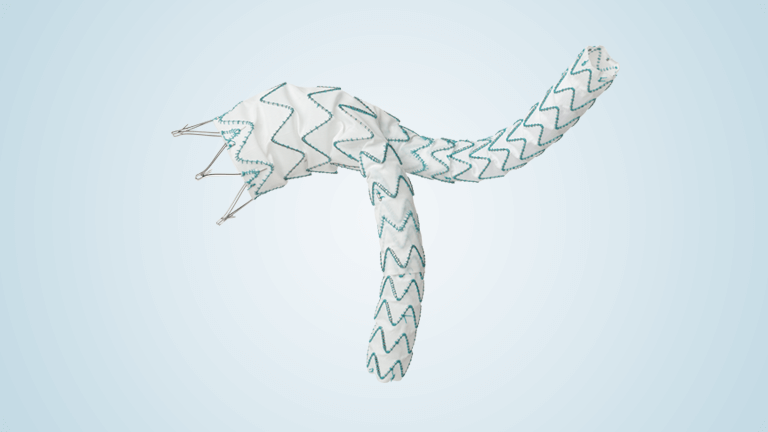
The TREO Abdominal Stent-Graft System is approved by the US Food and Drug Administration (FDA) to treat patients with abdominal aortic aneurysms. This is a prospective, multi-center, non-randomised, single-arm, post-market study; the purpose is to evaluate the real world, long-term performance of the device as a treatment for patients with infrarenal abdominal aortic aneurysms or […]
Terumo Aortic announces publication of the primary endpoint results from the TREO Pivotal Study

Following recent approval by the US Food and Drug Administration (FDA) of the TREO® Abdominal Aortic Stent-Graft System for the treatment of patients with abdominal aortic aneurysms (AAA), Terumo Aortic announced today the publication of the primary endpoint results from the investigational device exemption (IDE) pivotal study in the Journal of Vascular Surgery.
Terumo Aortic announces completion of enrolment of Relay®Pro US pivotal study
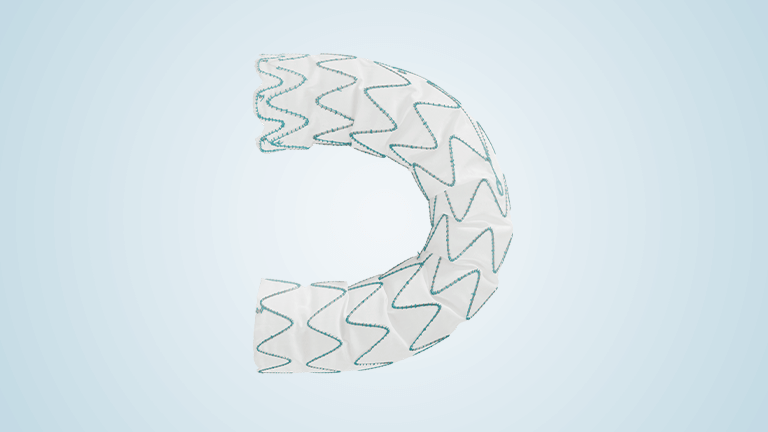
RelayPro is a low profile, next generation thoracic stent graft device designed to expand the treatment of thoracic endovascular aortic repair (TEVAR) to patients with smaller access vessels. RelayPro, having obtained CE Mark in 2018, utilises the same stent design, material and dual sheath technology already proven in Terumo Aortic’s Relay®Plus device, with the additional […]
Terumo Aortic announces launch of industry-first global endovascular registry

TiGER is a global, prospective, multi-arm, multi-centre registry evaluating more than 1,000 patients over a period of 5 years. The study will collect a broad range of clinical evidence on the company’s comprehensive thoracic and abdominal endovascular product portfolio. At the recent European Investigators’ Meeting, the TiGER Principal Investigator, Professor Vincent Riambau, Barcelona commented, “This […]