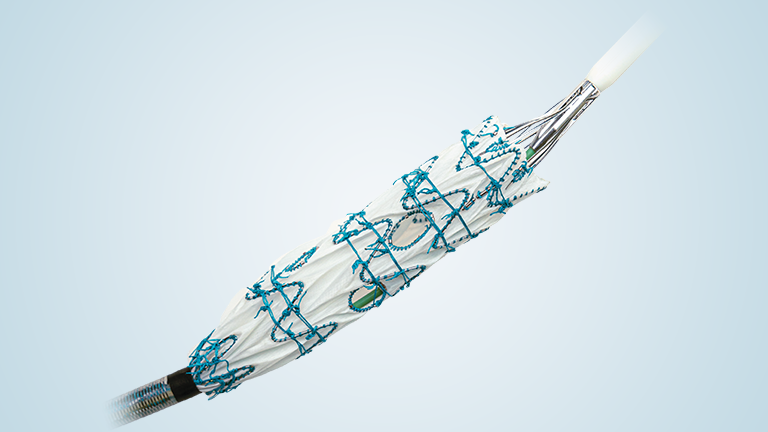
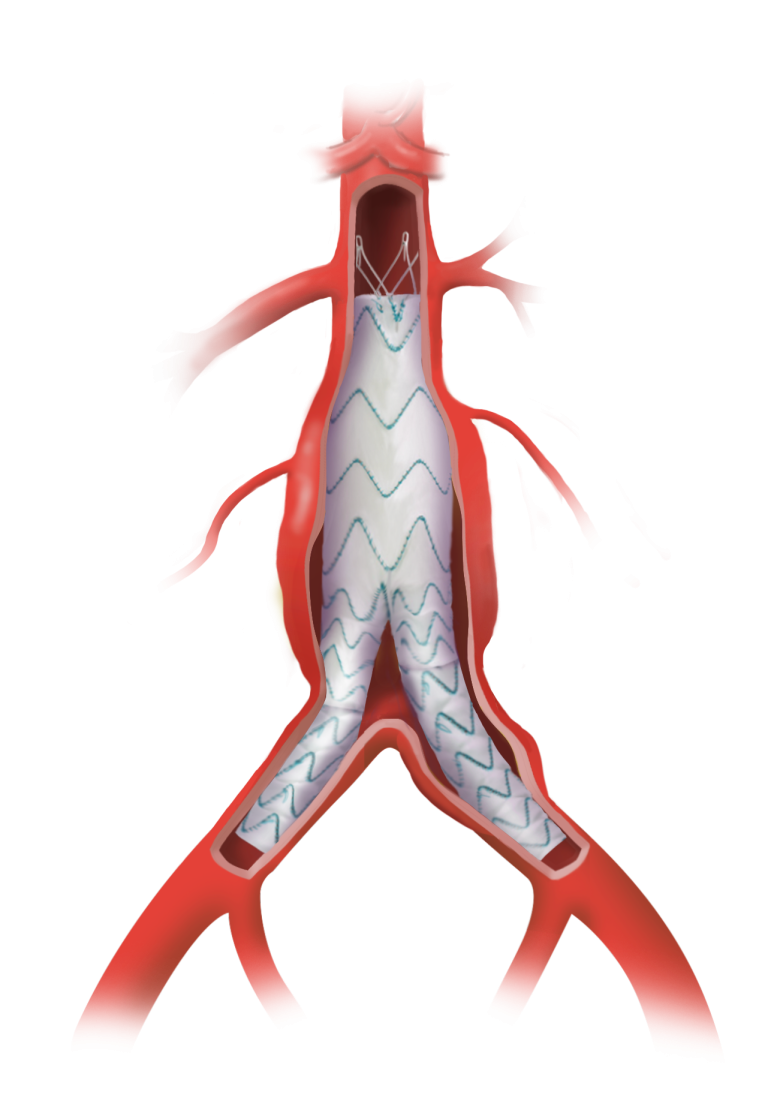
The study is designed to evaluate the endovascular repair of juxtarenal and suprarenal aortic aneurysms using the Fenestrated TREO® Abdominal Stent-Graft System. Getinge’s iCast® covered stent system will be used as a bridging stent in this clinical study*.
Achieving the first enrolment in this FDA approved IDE is a major milestone that brings us one step closer to making a fenestrated endovascular graft system available to patients in the US, representing a significant advancement in treating complex abdominal aortic aneurysms (AAA).
The study will include up to 45 sites at leading healthcare institutions in the US. We anticipate that approximately 210 patients will be enrolled across three study arms, representing juxtarenal aneurysms, suprarenal aneurysms, and patients with chronic kidney disease. Patients will then be followed for 5 years post procedure.
The first patient was enrolled by J. Westley Ohman, MD, FACS, FSVS and Director of Vascular Surgery at Washington University School of Medicine.
The National Principal Investigator, Benjamin Starnes, MD, FACS, and Alexander Whitehill Clowes, Endowed Chair in Vascular Surgery and Professor at Harborview Medical Center commented, “The Fenestrated TREO® Stent-Graft System provides a revolutionary technology that is not available with any other Endovascular graft in the US today. I believe this product will become the gold standard for treating juxtarenal and suprarenal aneurysms.”
Jeffrey Mifek, Global Vice President, Clinical and Medical Affairs at Terumo Aortic said: “The Fenestrated TREO® Stent-graft system has now been implanted in more than 1,200 patients outside the US, and today we are delighted to mark the first implant of this device in our US Pivotal Study. The US IDE system can be manufactured with up to 5 fenestrations. This capability expands the options available for tailoring grafts to individual patient anatomies within the US market. Terumo Aortic is committed to advancing aortic care and to delivering a solution for every patient.”
*CAUTION—INVESTIGATIONAL DEVICE. Limited by Federal law to investigational use