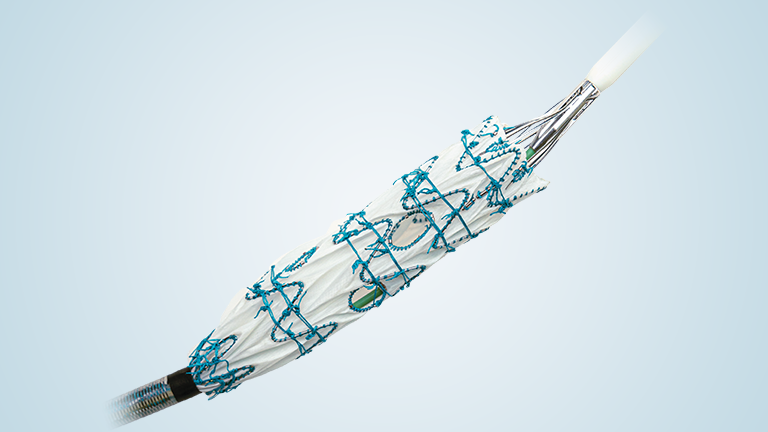
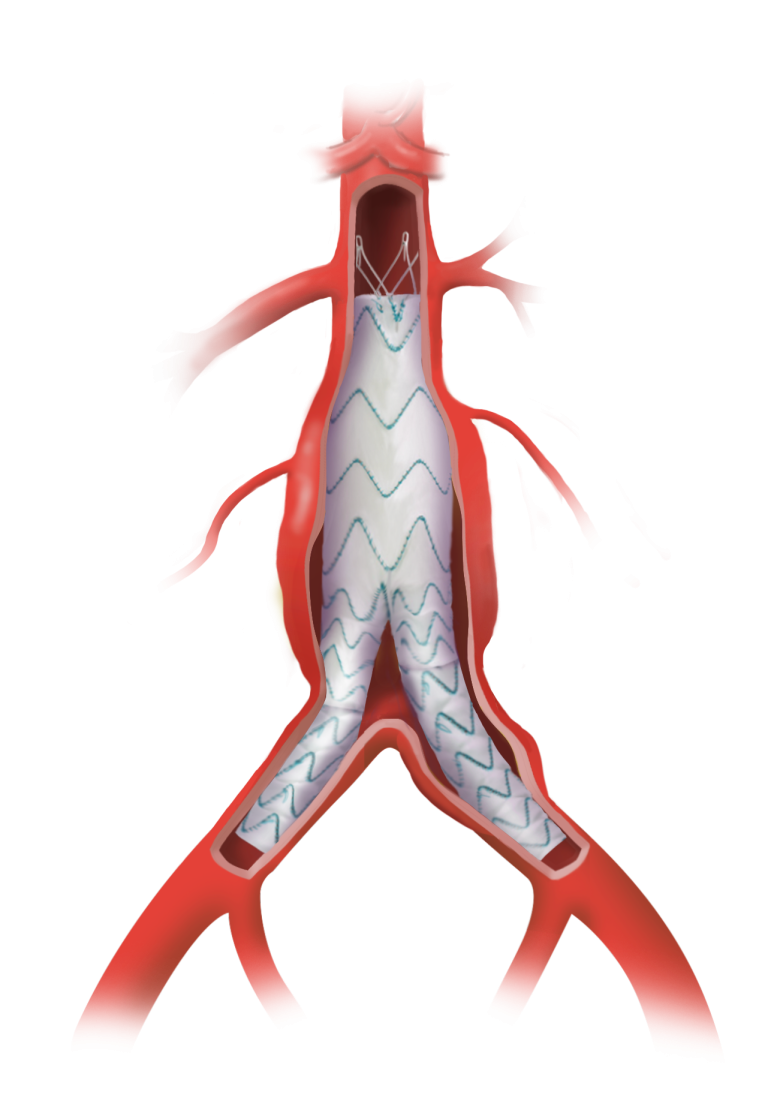
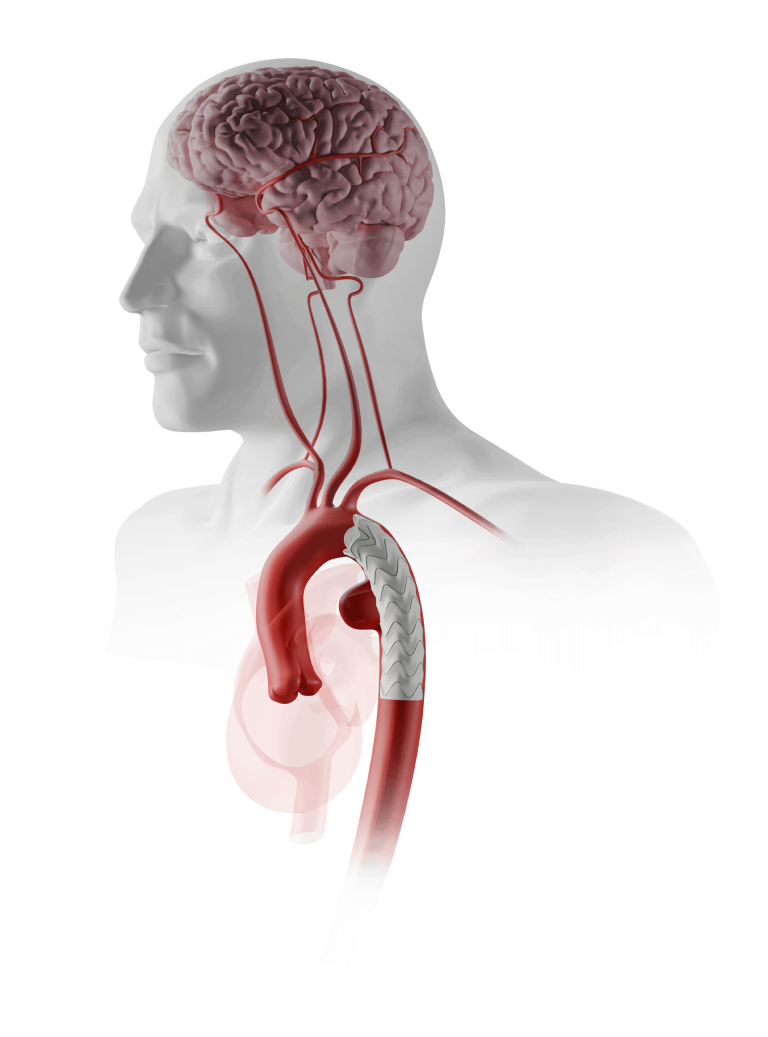
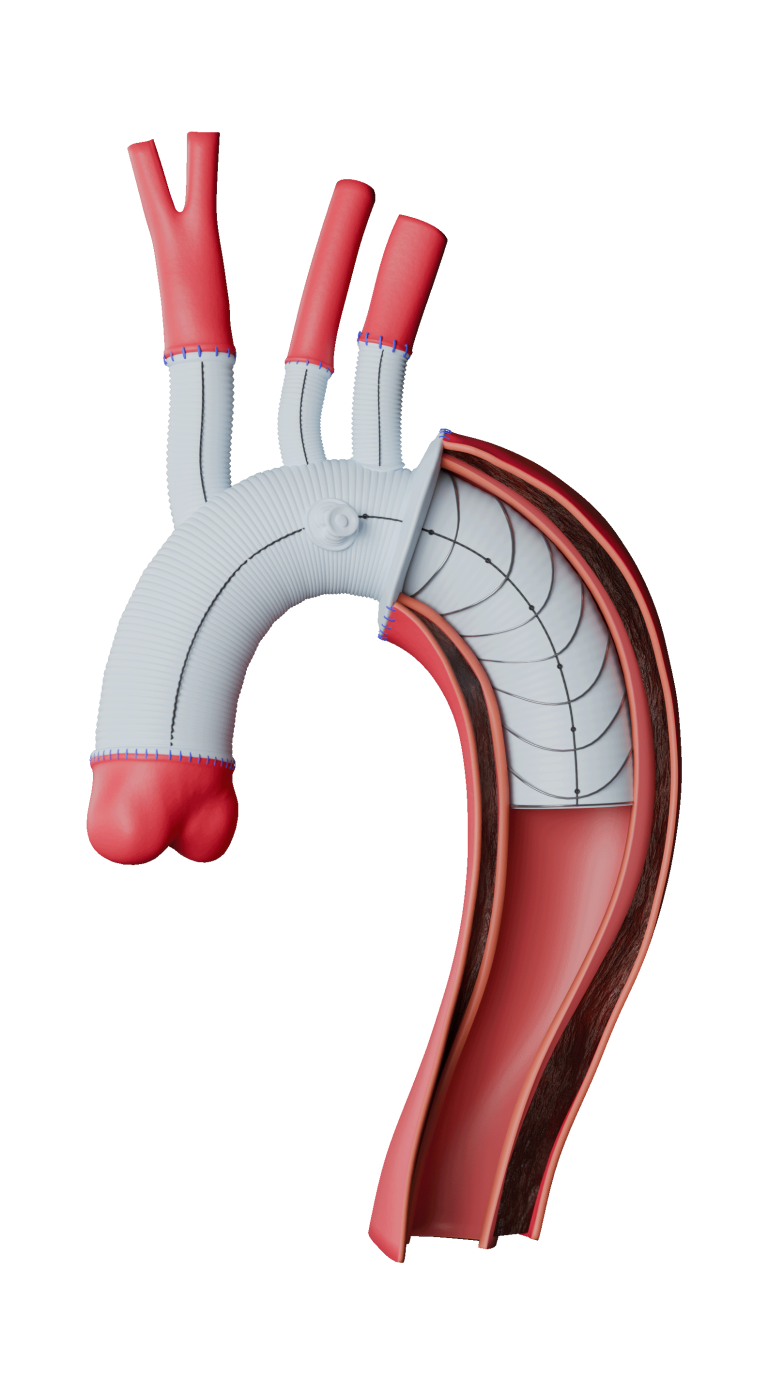
TiGER is a global, prospective, multi-arm, multi-centre registry evaluating more than 1,000 patients over a period of 5 years. The study will collect a broad range of clinical evidence on the company’s comprehensive thoracic and abdominal endovascular product portfolio.
At the recent European Investigators’ Meeting, the TiGER Principal Investigator, Professor Vincent Riambau, Barcelona commented, “This is a truly unique registry encompassing the complete range of endovascular solutions for the entire aorta. This will provide an unparalleled perspective on the ‘real world’ results of aortic endovascular procedures.”
The registry will begin enrolling patients across approximately 40 sites in Europe with plans to expand into additional geographies including the United States, Japan and Latin America.
Mark Miles, Chief Commercial Officer of Terumo Aortic added, “TiGER is the industry’s first global registry evaluating patients across multiple aortic pathologies using both standard and custom endovascular technologies. This reinforces our commitment to transforming the treatment of aortic disease and serves as the model for an evidence-based approach required for broad adoption of innovative device solutions.”
The launch of the TiGER Registry marks an important milestone for Terumo Aortic as it continues to expand its surgical, endovascular and hybrid businesses worldwide.