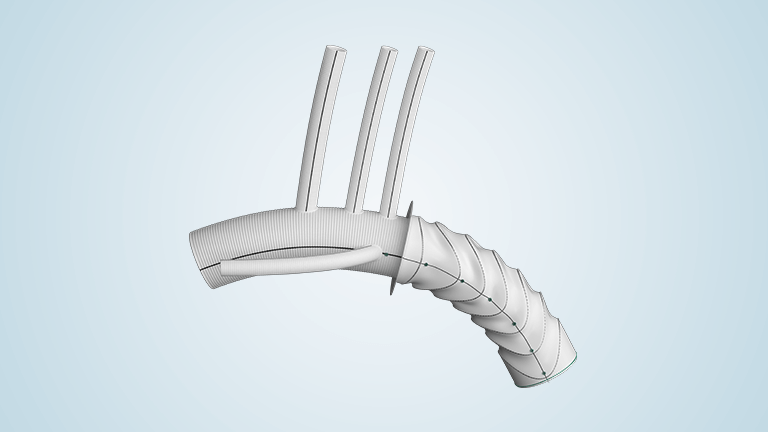
Relay®Pro delivers uncompromised clinical performance in the lowest commercially available profile and is indicated for all pathologies of the descending thoracic artery; aneurysm, penetrating atherosclerotic ulcers, transection and dissection. Relay®Pro offers unparalleled accuracy, proven efficacy and durability as well as the widest range of sizes, tapering and configurations on the market, designed to expand the treatment of thoracic endovascular aortic repair (TEVAR) to patients with smaller access vessels.
This approval follows the excellent results from recent studies on Relay®Pro Dissection (RelayPro-D) and Relay®Pro traumatic injury (RelayPro-T).
The Pro-D study was a prospective, multicenter pivotal trial conducted in 22 US centers and enrolled 56 patients with acute, complicated Type B aortic dissection. Approximately two thirds of the subjects had dissection extension to the iliac arteries. The primary endpoint of all-cause mortality at 30 days was 1.8% and demonstrated a clear early survival benefit. Patients will be followed for 5 years.
National Principal Investigator of the Pro-D study, Peter Rossi, MD, FACS, DFSVS, Professor and Chief of the Division of Vascular and Endovascular Surgery at the Medical College of Wisconsin, said: “The fully covered, non-bare stent (NBS) configuration of Relay®Pro was most used in the pivotal trial, highlighting the clinical value of this platform, and a full 86% of procedures were percutaneous. I am very encouraged by the one-year outcomes of this pivotal study which will be published soon now that approval of this new generation device has been granted; also, it is heartening that our dissection patients will have this additional option.”
The Pro-T study was a prospective, multicenter pivotal study that enrolled 50 patients in 16 US centers. The purpose of the study was to assess the performance of Relay®Pro for the treatment of transection. The primary endpoint of the trial was all-cause mortality at 30 days was 2% with follow-up continuing for five years.
National Principal Investigator of the Pro-T study, Benjamin W Starnes MD, Professor and Chief of Vascular Surgery, University of Washington, Seattle, commented: “We’re looking forward to having this latest-generation device available for all thoracic indications. A low-profile device will be particularly useful for trauma patients who are typically younger and with smaller access vessels. 80% of the procedures in this study were percutaneous, highlighting a distinct value of the Relay®Pro stent-graft system.” The approval of RelayPro-T also introduces a 22mm diameter option.
Furthermore, with the Relay®Pro “Upon Request” program, there are approximately 2000 stent-graft configurations approved by the FDA; this truly comprehensive portfolio allows physicians unparalleled choice and versatility allowing physicians to select devices that best fit the anatomical needs of each individual patient.
The Relay®Pro Thoracic Stent Graft received CE Mark approval in 2017 and FDA approval in 2021 for the treatment of patients with Thoracic Aortic Aneurysms (TAA) and Penetrating Atherosclerotic Ulcers (PAUs).