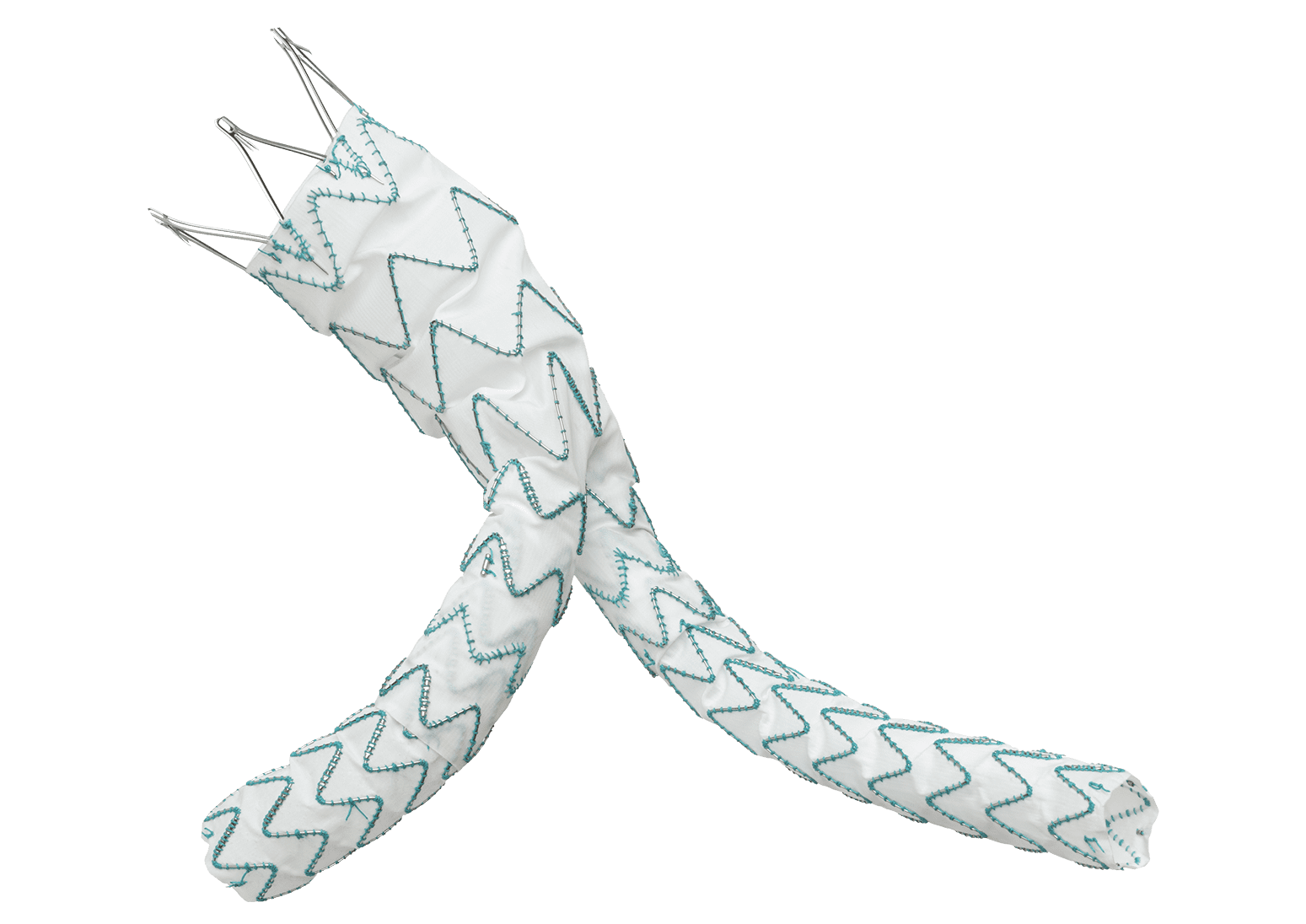
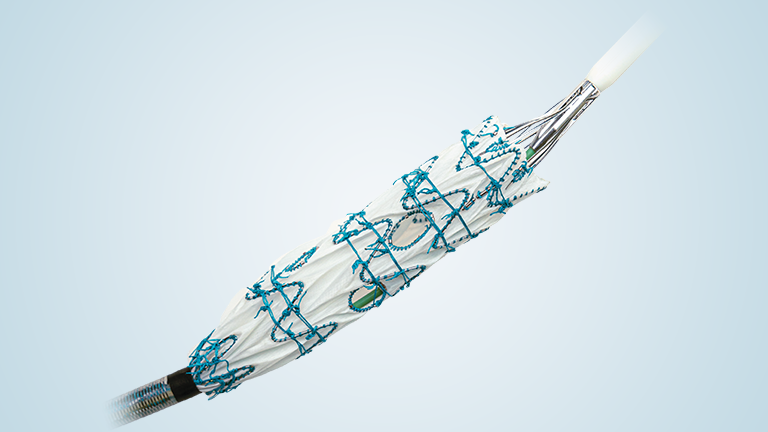
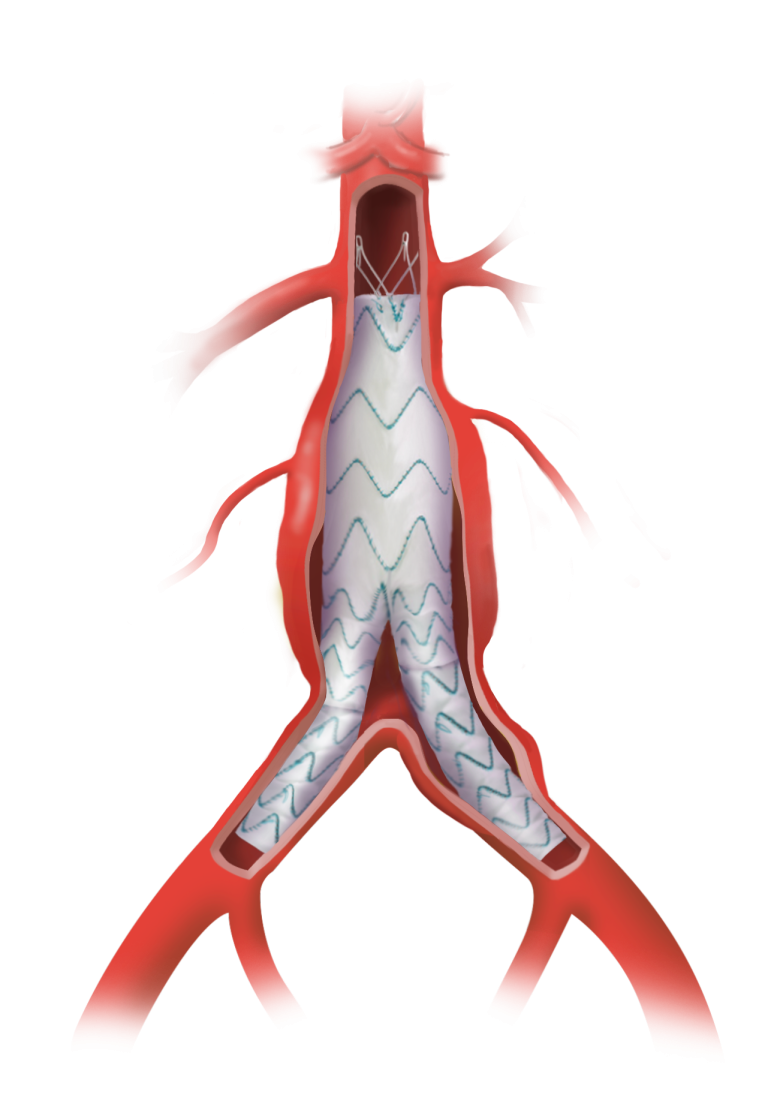
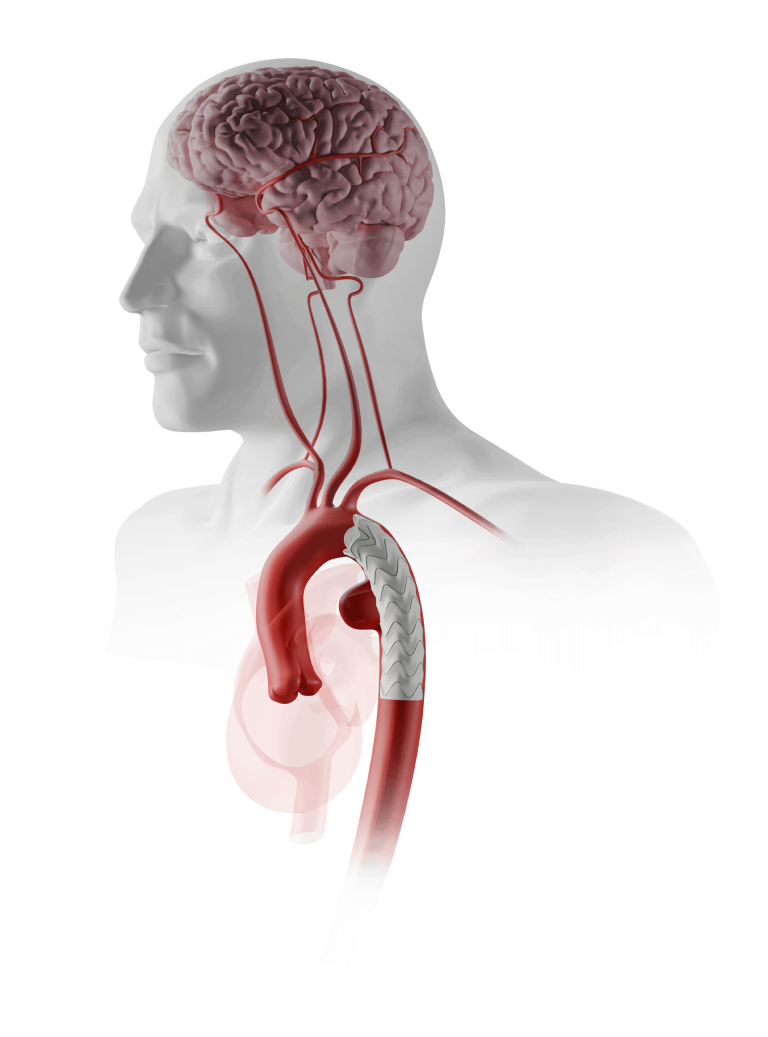
The TREO Abdominal Stent-Graft System is approved by the US Food and Drug Administration (FDA) to treat patients with abdominal aortic aneurysms. This is a prospective, multi-center, non-randomised, single-arm, post-market study; the purpose is to evaluate the real world, long-term performance of the device as a treatment for patients with infrarenal abdominal aortic aneurysms or aorto-iliac aneurysms.
Enrolment has now passed the 50% milestone to recruit a minimum of 300 patients who have had the TREO Abdominal Stent-Graft System implanted at up to 55 investigational sites in the United States. All patients will be followed for 5 years.
As the leading study enroller and Principal Investigator, Jeromy Brink, MD, Vascular and Endovascular Surgeon, Banner University Medical Center, Phoenix, Arizona commented: “The all-comers scope of this study compared with the pre-market study provides the opportunity to review data from a much larger patient population resulting in a far better understanding of the device and how it performs over an extended period of time.”
Jeffrey Mifek, Global Vice President, Clinical and Medical Affairs at Terumo Aortic added: “Over the next few years, the primary objective of this study is to collate real world safety and effectiveness data of TREO and provide insights into long-term performance. The data collected may allow for expanded indications of this device to treat a wider group of patients.”
TREO received CE Mark approval in 2015, FDA approval in 2020 and PMDA approval in 2021. This device is integral to Terumo Aortic’s innovative portfolio of surgical, endovascular and hybrid devices to treat every segment of the aorta.