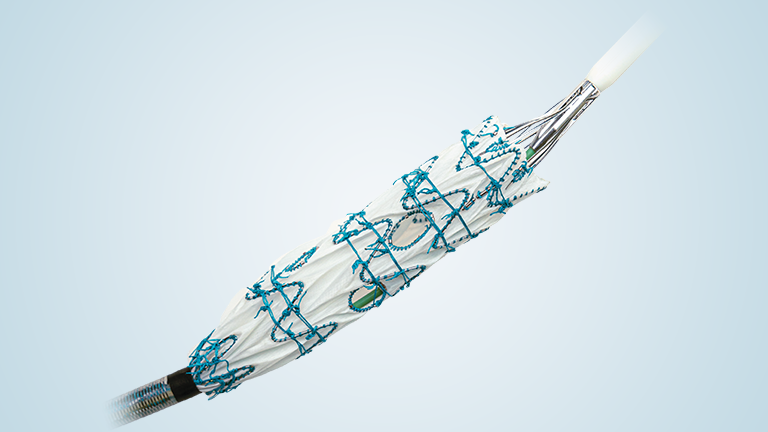
RelayPro is a low profile, next generation thoracic stent graft device designed to expand the treatment of thoracic endovascular aortic repair (TEVAR) to patients with smaller access vessels.
RelayPro, having obtained CE Mark in 2018, utilises the same stent design, material and dual sheath technology already proven in Terumo Aortic’s Relay®Plus device, with the additional benefit of a 3 to 4 Fr reduction in outer profile.
The RelayPro aneurysm study is a prospective, multi-centre, non-blinded, non-randomised study of the RelayPro thoracic stent graft in patients with thoracic aortic aneurysms and penetrating atherosclerotic ulcers. The primary endpoint for safety is a composite of major adverse events; the primary endpoint for effectiveness is a composite of technical and clinical success. One-hundred and ten patients have been enrolled in the study across sites in both the United States and Japan.
Dr Wilson Szeto (Cardiothoracic Surgeon, University Medical Centre of Pennsylvania) Co-Principal Investigator, commented, “RelayPro’s ability to land accurately combined with its low profile will allow me to successfully treat complex anatomy with precision.”
This trial marks the first time both Bare Stent and Non-Bare Stent (NBS) configurations of RelayPro have been used in the United States.
Dr Venkatesh Ramaiah (Vascular Surgeon, HonorHealth Medical Group, Arizona) Co-Principal Investigator, stated, “One of the key benefits of RelayPro is being able to choose from a range of proximal configurations allowing me to tailor my device selection to meet the individual needs of each patient.”
The completion of enrolment is an important milestone for Terumo Aortic as the company progresses towards the approval of RelayPro in the United States and Japan.