Today, Terumo Aortic, a global medical device company dedicated to developing solutions for aortic disease, and Bentley, a leading global manufacturer of balloon-expandable covered stents, announced their partnership in a clinical study in the United States. The objective is to obtain US Food and Drug Administration (FDA) Investigational Device Exemption (IDE) approval for Terumo Aortic’s Fenestrated TREO® device, in combination with Bentley’s BeFlared FEVAR Stent Graft System for Fenestrated Endovascular Aneurysm Repair (FEVAR) procedures. The IDE submission is planned for the first half of 2026.
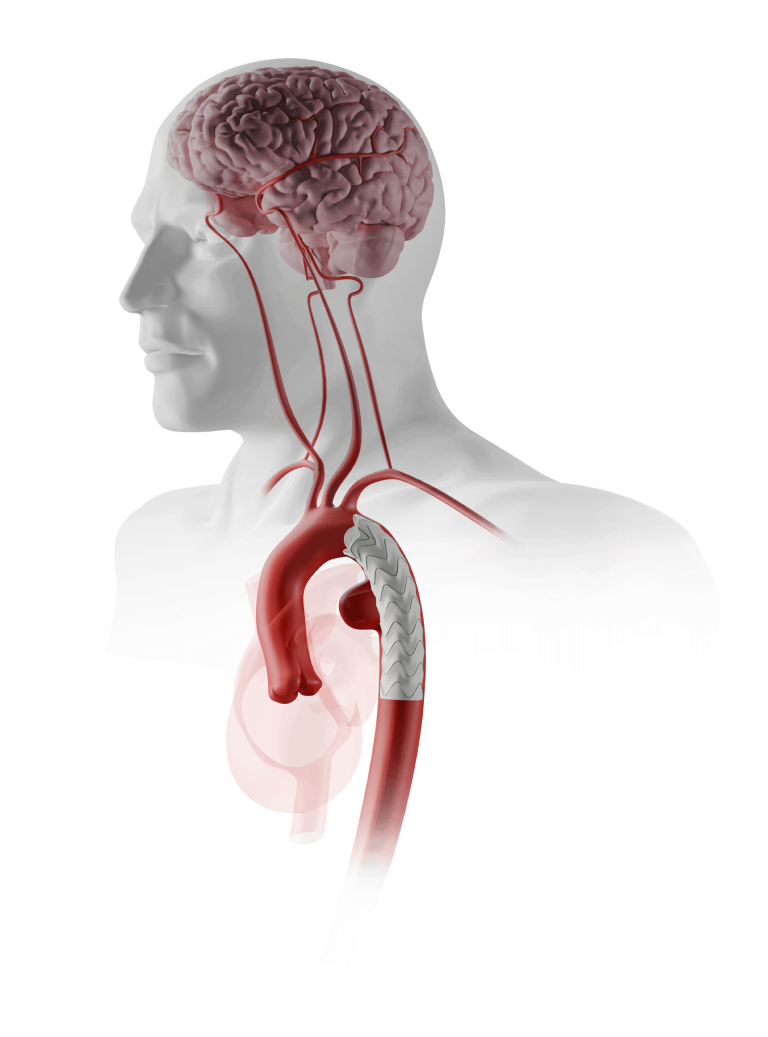
Through this collaboration, Terumo Aortic and Bentley aim to bring their innovative solutions to benefit US patients suffering from complex abdominal aortic aneurysms, namely juxtarenal and suprarenal aneurysms. Following the completion of the joint clinical study and FDA approval, both companies plan to independently market their products in the US.
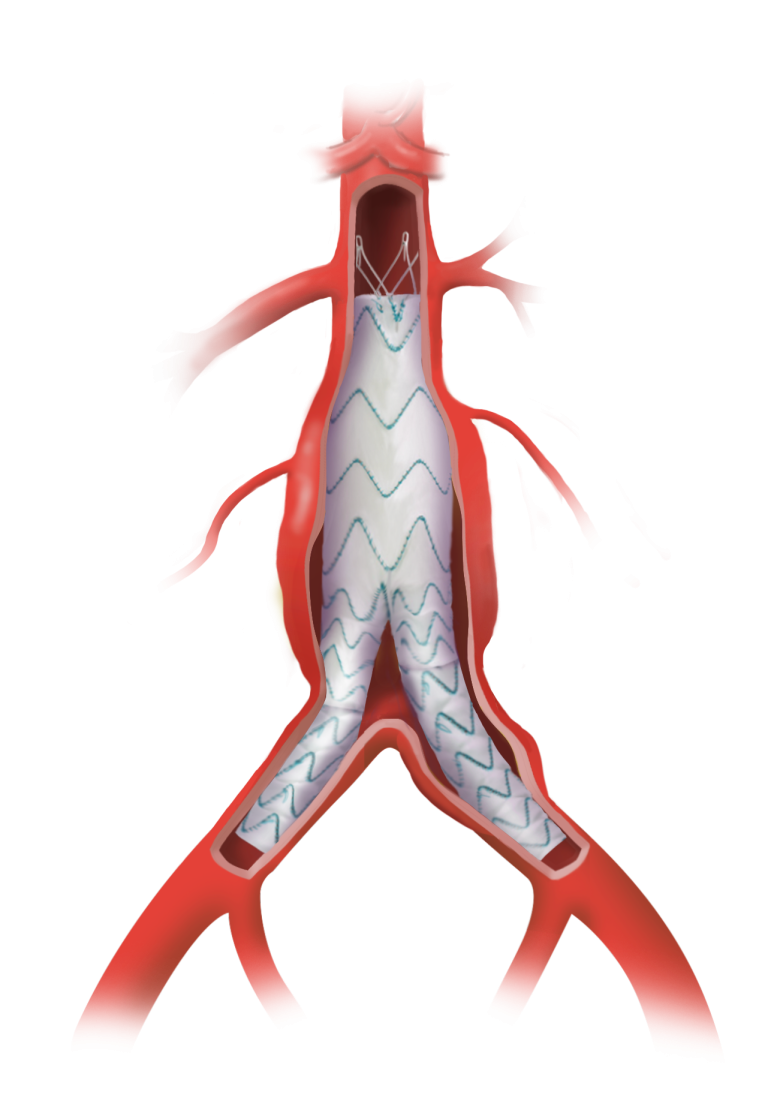
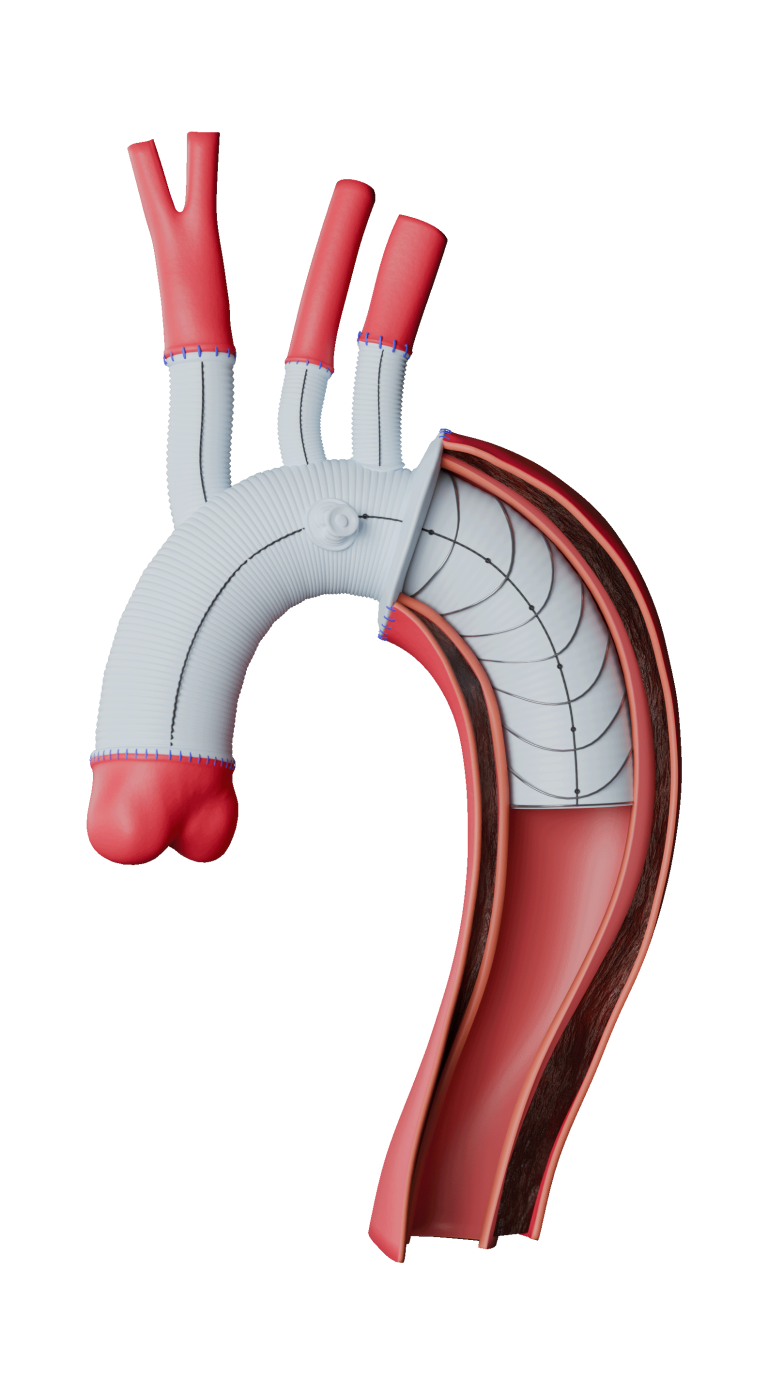
Terumo Aortic’s Fenestrated TREO® device offers a patient-specific solution tailored to the anatomical conditions and clinical needs for treating aortic aneurysms. To ensure perfusion of vital organs, the Fenestrated TREO® device can be designed to include up to five fenestrations, requiring reliable bridging stents to maintain perfusion to the target vessels.
Erik Pomp, CEO of Terumo Aortic commented “Bentley’s BeFlared, the world’s first-to-market dedicated bridging stent, is a great fit for our Fenestrated TREO® platform. With its innovative features, this stent has the potential to become the gold standard in FEVAR procedures, thus complementing our Fenestrated TREO® platform.”
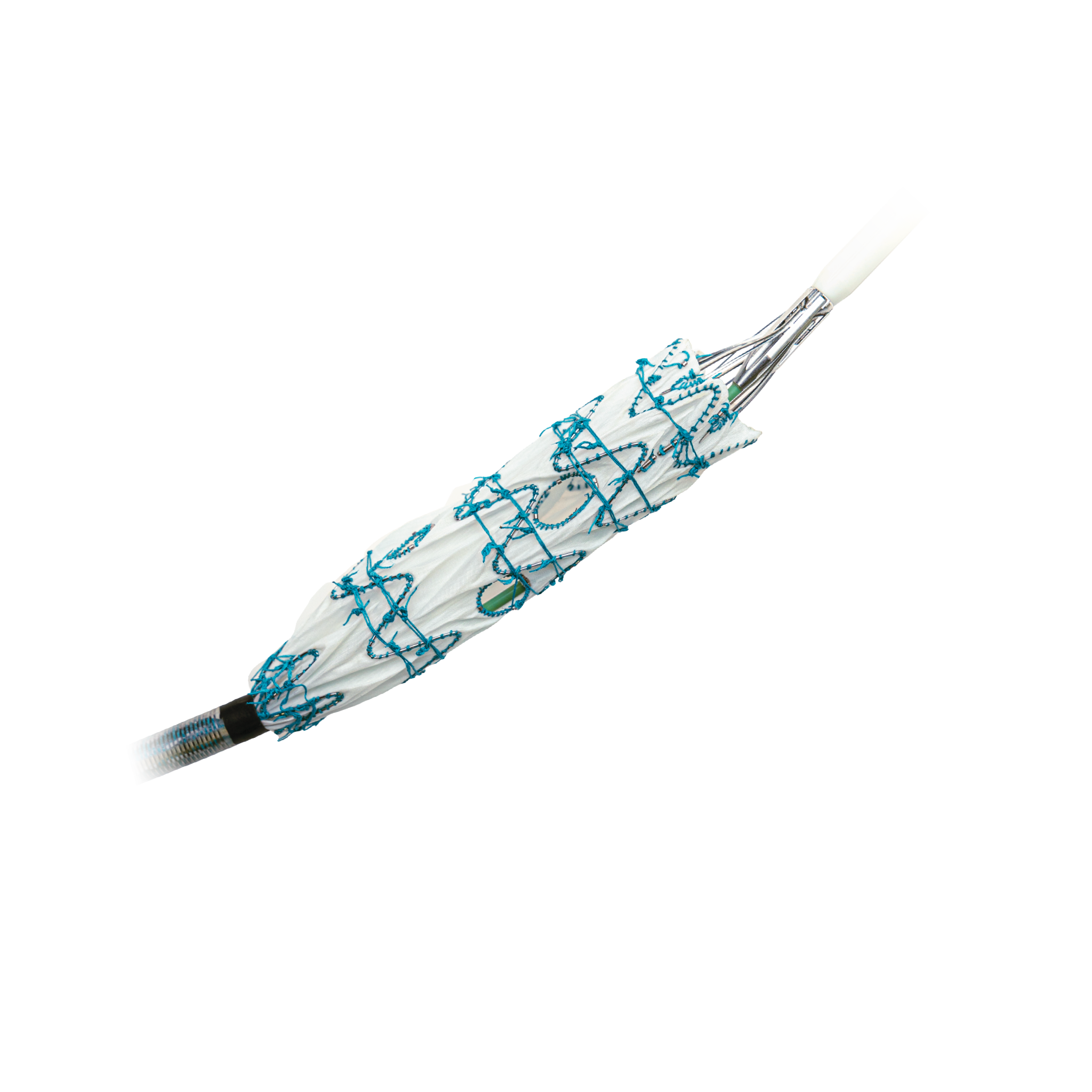
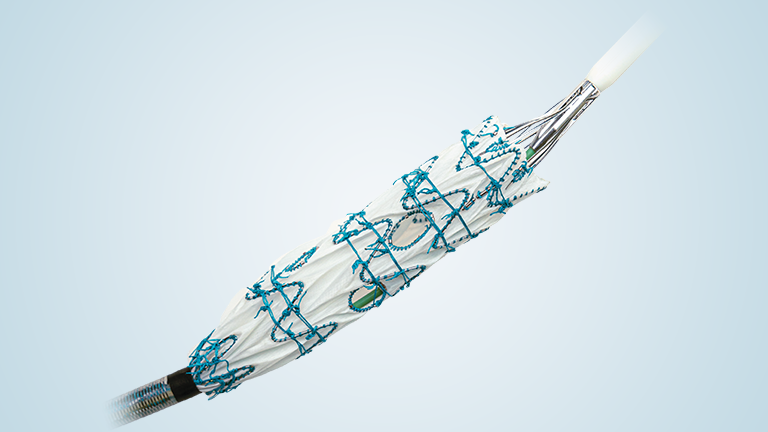
BeFlared is the world’s first bridging stent specifically developed for FEVAR procedures. The stent is crimped on a specially designed stepped balloon which will reduce the number of steps that are needed for optimal deployment. Additionally, a third radiopaque marker aids in optimal positioning of the stents in the fenestration. Having already received CE certification from TÜV Süd, BeFlared is now available in most key markets. The “first in human” procedure was performed by Professor Stéphan Haulon in Paris in November 2024.
“By partnering with Terumo Aortic, we’re advancing BeFlared’s role as the go-to bridging stent for FEVAR. Already available in most key markets, its value is being demonstrated through strong outcomes led by KOLs. This study will further solidify its impact in treating complex aortic aneurysms. Terumo Aortic’s Fenestrated TREO® platform is an excellent complement, and together, we are setting new standards in patient care” said Sebastian Büchert, CEO of Bentley.
The combination of Fenestrated TREO® and BeFlared is designed to set a new benchmark for FEVAR procedures by offering both ease of use and reduced complications for patients. Together, Terumo Aortic and Bentley are driving innovation in the field of aortic disease treatment.