Innovation through Collaboration
Custom Solutions
Caution: Not available in the USA
The availability of custom-made devices is subject to local regulatory guidelines.
Custom Solutions
The availability of custom-made devices is subject to local regulatory guidelines.
Caution not available in the USA
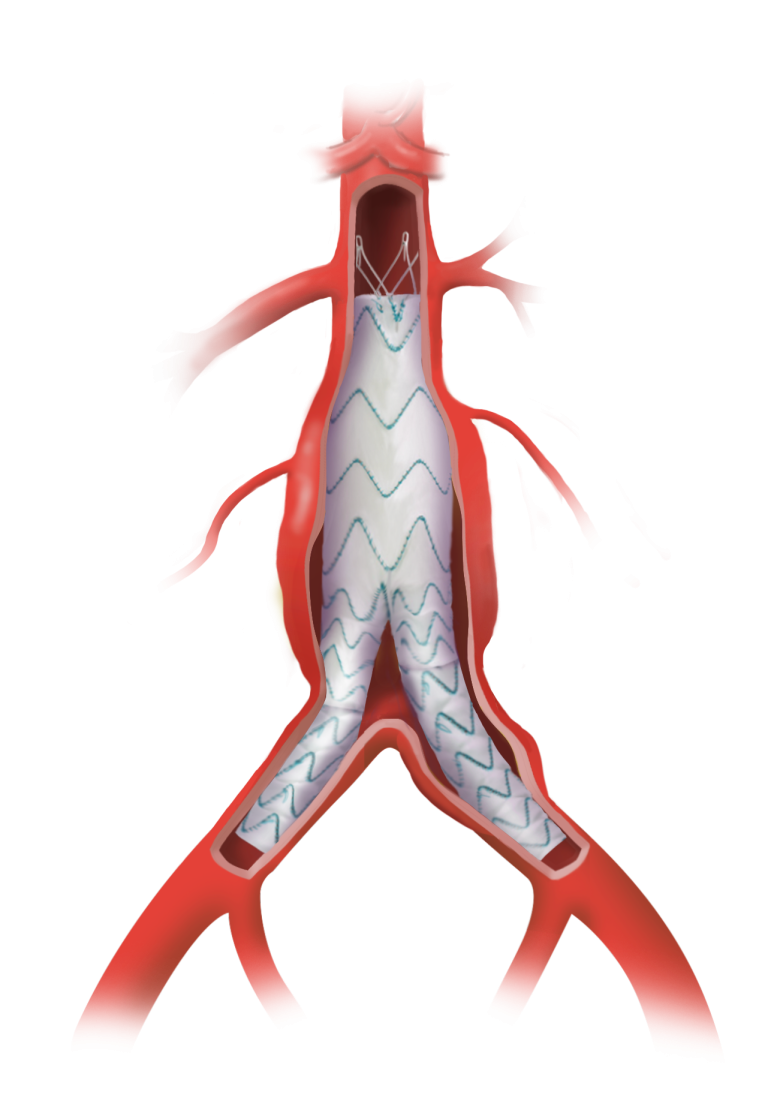
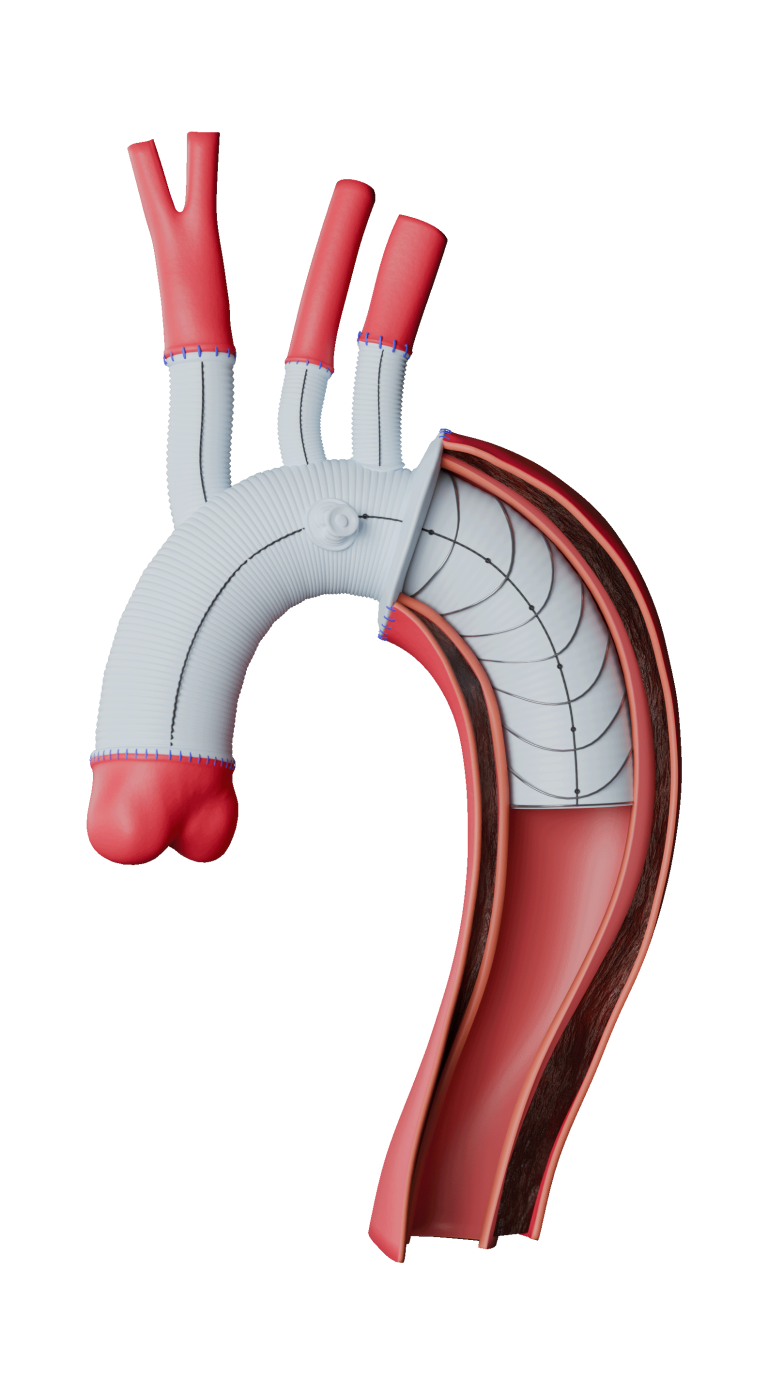
Custom
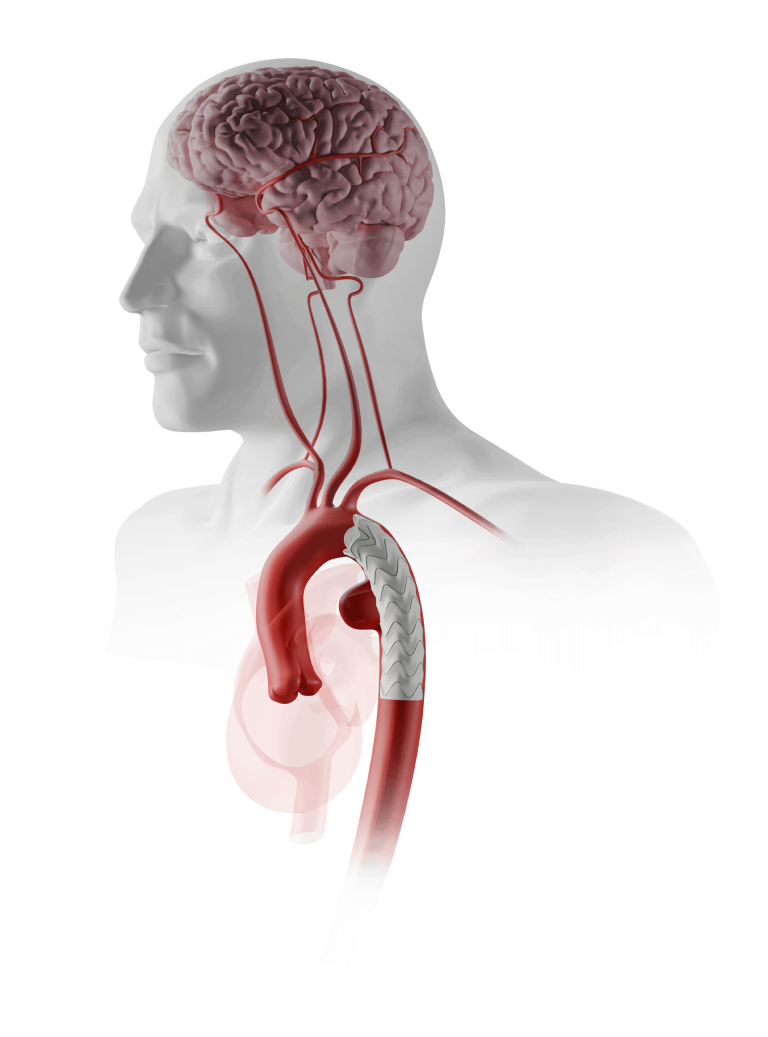
Relay®Branch
We are committed to delivering the best solution to meet the needs of your patient and your practice.
Our dedicated team of custom device specialists works with you to develop solutions for even the most complex of anatomies.
Communication
Assistance
Delivery
Case Manager
Product Disclaimer
Custom made devices are specifically made in accordance with a written prescription of any person authorised by national law by virtue of that person’s professional qualifications; which gives (1) specific design characteristics provided under that person’s responsibility and (2) is intended for the sole use of a particular patient exclusively to meet their individual conditions and needs.
Custom made devices are not available in the US and availability is subject to local regulatory approval.
Instructions for Use
An IFU is provided with each custom device.
View the eIFU for more information on use, indications, contraindications, warnings/precautions and availability within your market.
Discover our technology
Our products are powered by technologies that help ensure that every product you use can be implanted or deployed safely.