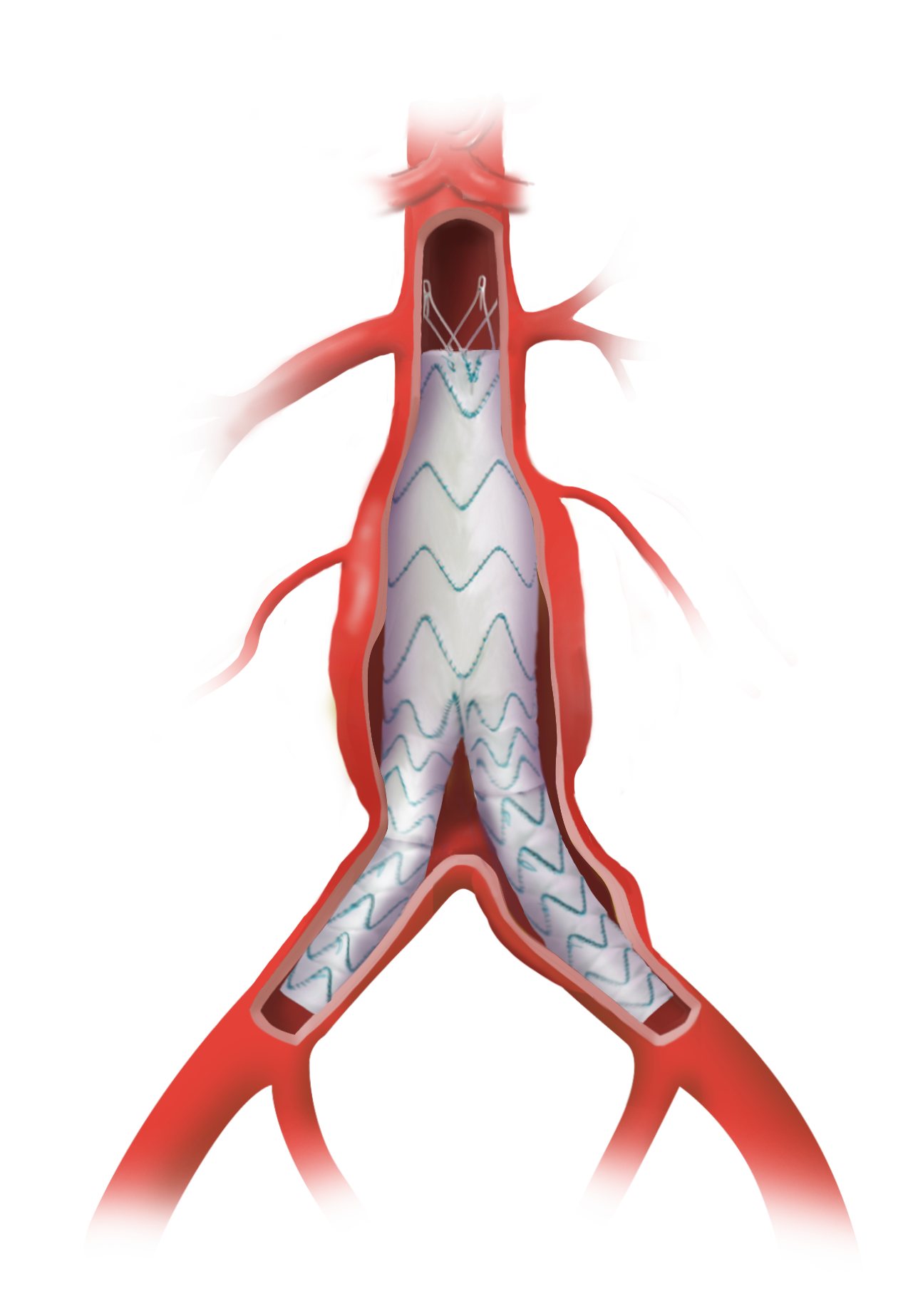
Terumo Aortic announces US FDA Approval for TREO endovascular device
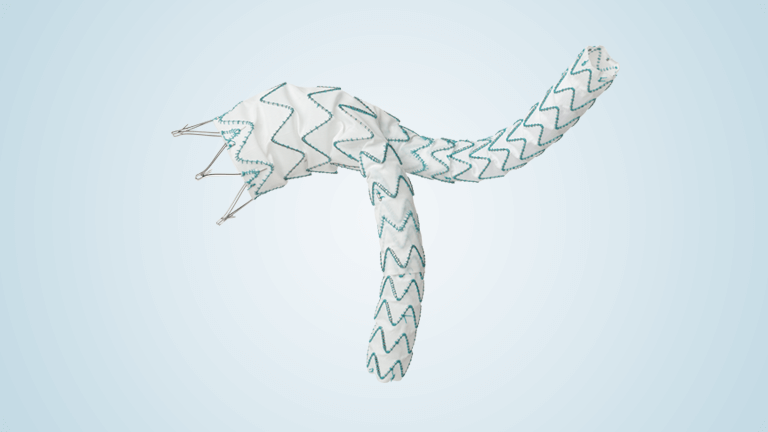
TREO is the only device commercially available for endovascular aneurysm repair (EVAR) with dual proximal fixation and lock stent technology offering physicians the next advancement in endovascular device solutions. It is a three-piece design featuring in situ limb adjustability and provides a wide range of aortic device configurations to specifically address the anatomy of each […]
Terumo Aortic Announces US FDA Breakthrough Device designation for Thoraflex Hybrid device
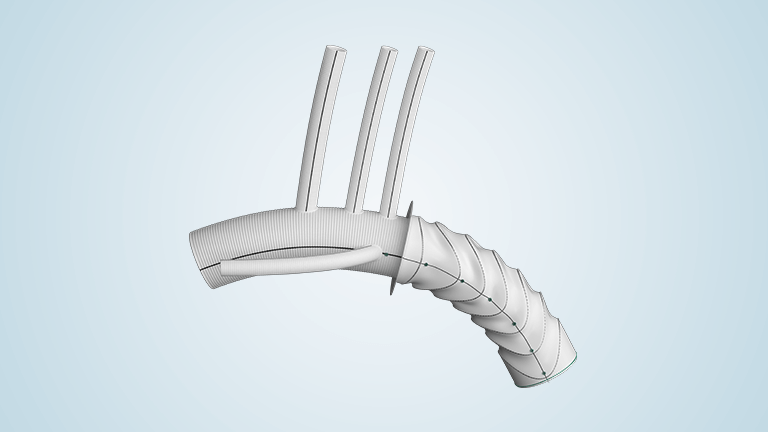
The purpose of the FDA’s Breakthrough Device Designation program is to fast-track the regulatory review process for certain medical technologies and device-led combination products that satisfy certain criteria; these include providing a more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions. The aim of the program is to provide patients and […]