Terumo Aortic announces publication of the primary endpoint results from the TREO Pivotal Study
Following recent approval by the US Food and Drug Administration (FDA) of the TREO® Abdominal Aortic Stent-Graft System for the treatment of patients with abdominal aortic aneurysms (AAA), Terumo Aortic announced today the publication of the primary endpoint results from the investigational device exemption (IDE) pivotal study in the Journal of Vascular Surgery.
Terumo Creates Global Group Music

The composition is based on the concept “Let’s respect the past, dream for the future, and open new doors together.” The track is composed by Mr. Akira Senju, a highly regarded Japanese composer and recipient of numerous awards and honors. Mr. Senju’s 35th anniversary album which was released on October 21 includes ‘TOGETHER’, one of […]
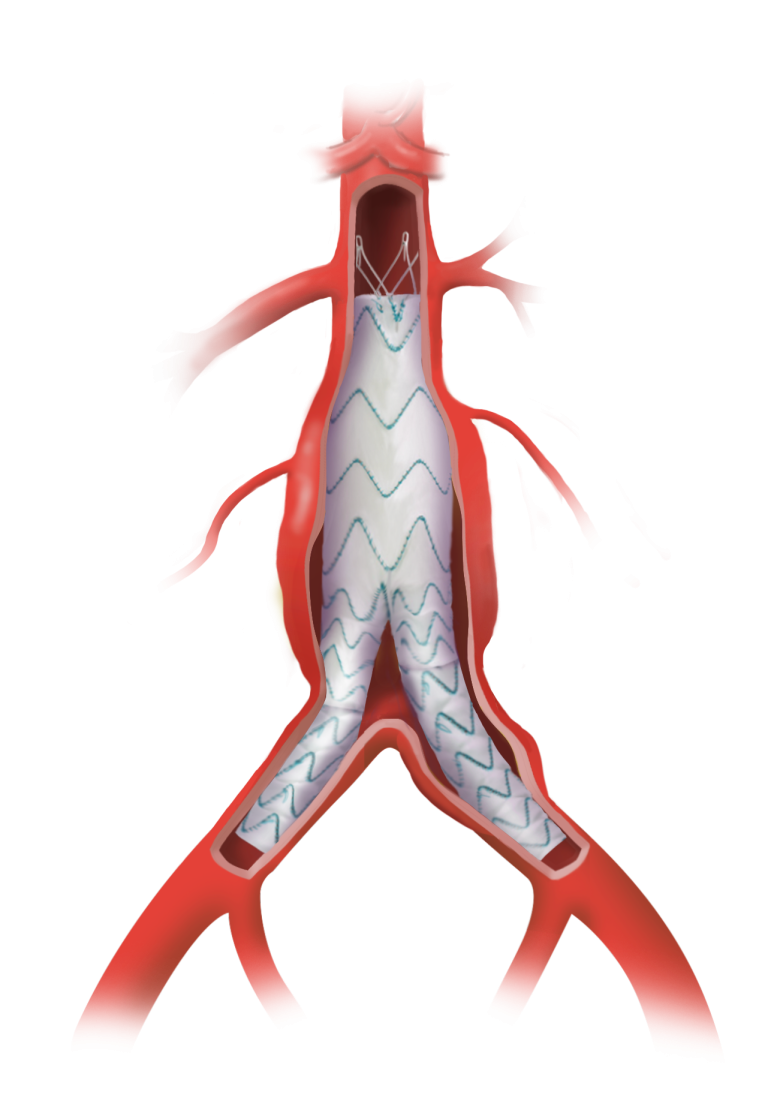
Terumo Aortic announces US FDA Approval for TREO endovascular device

TREO is the only device commercially available for endovascular aneurysm repair (EVAR) with dual proximal fixation and lock stent technology offering physicians the next advancement in endovascular device solutions. It is a three-piece design featuring in situ limb adjustability and provides a wide range of aortic device configurations to specifically address the anatomy of each […]
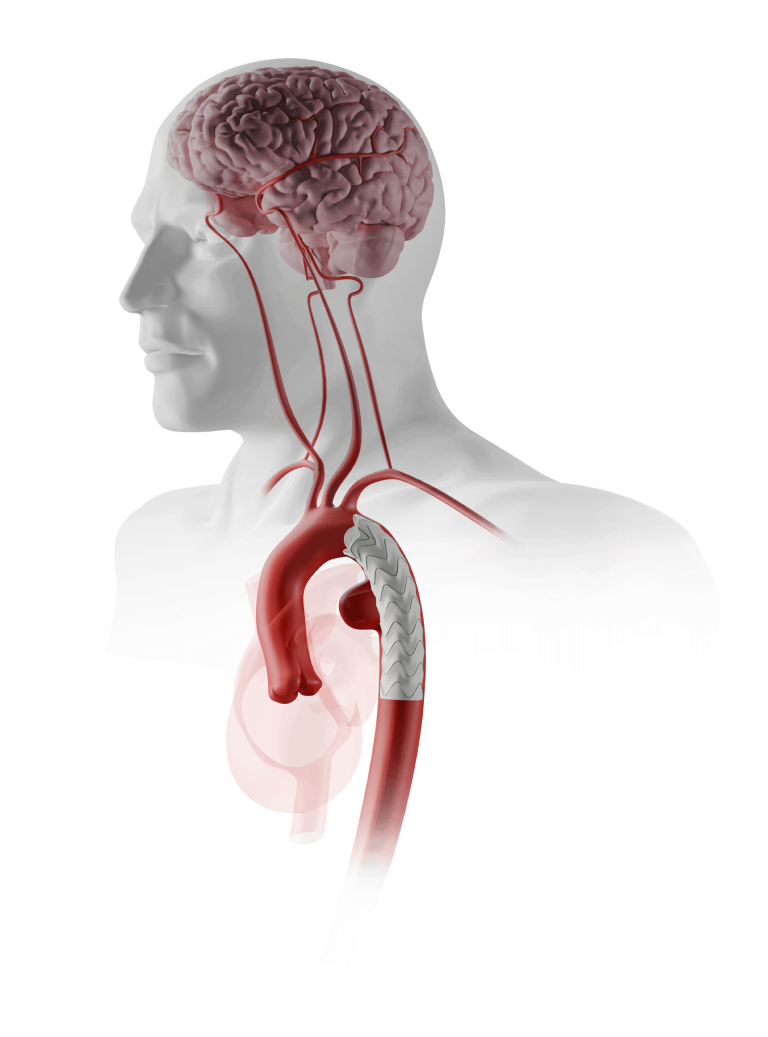
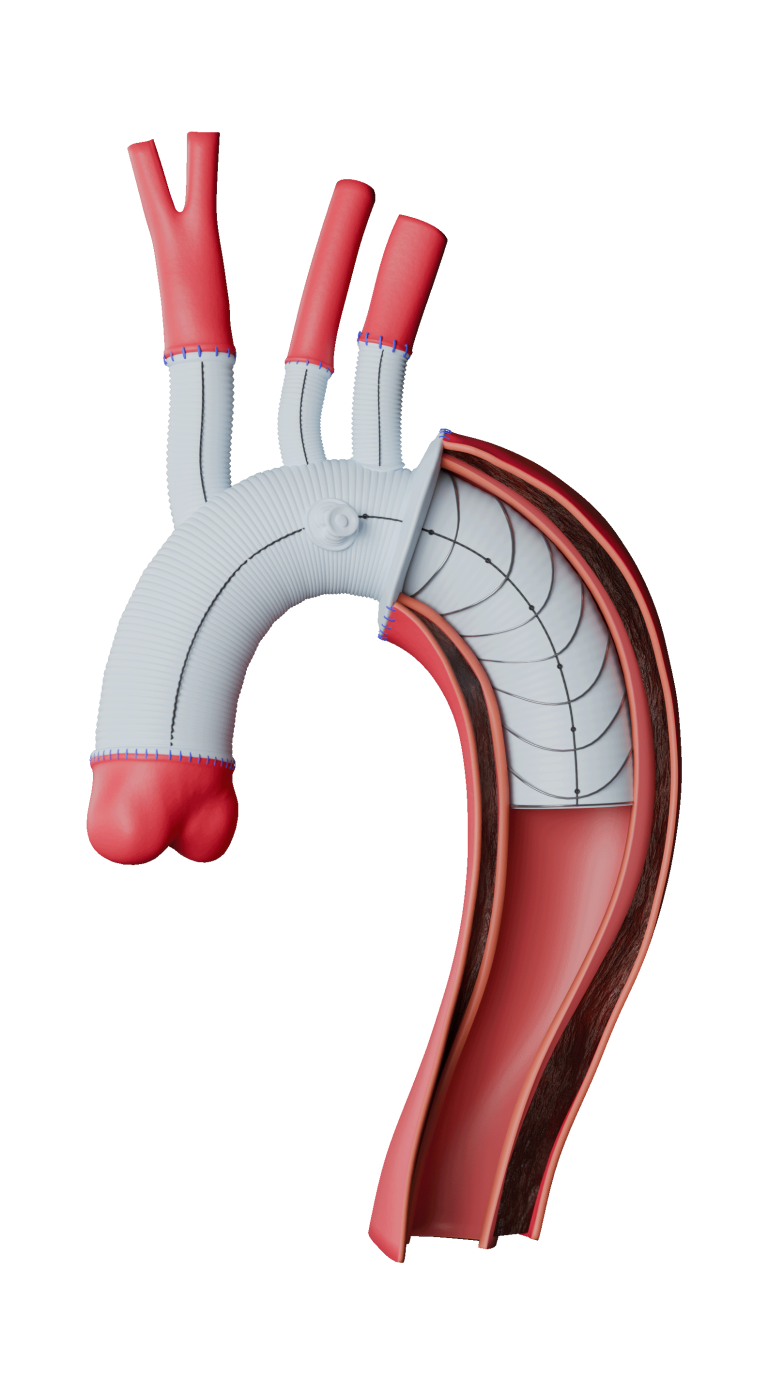
Terumo Aortic Announces US FDA Breakthrough Device designation for Thoraflex Hybrid device
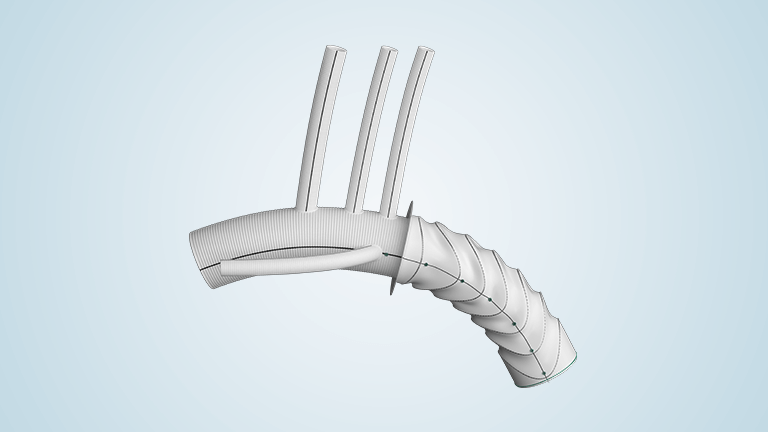
The purpose of the FDA’s Breakthrough Device Designation program is to fast-track the regulatory review process for certain medical technologies and device-led combination products that satisfy certain criteria; these include providing a more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions. The aim of the program is to provide patients and […]
Terumo Donates USD 2.4 Million in Cash and Products to Support COVID-19 Pandemic Relief Efforts Worldwide

TOKYO, JAPAN – April 22, 2020 – As the world joins together to support healthcare workers and others on the front lines providing critical care to patients, Terumo Corporation (TSE: 4543) is donating USD 2.4 million in cash and products to support the novel coronavirus disease (COVID-19) relief efforts worldwide – including a USD 1 […]
Terumo acquires Aortica Corporation

Terumo Corporation (TSE: 4543) today announced that it has decided to acquire Aortica Corporation, a U.S.-based company dedicated to advancing the science of personalised vascular therapy. Aortica has developed an automated case planning software known as AortaFit™, designed to precisely match fenestrations on an endograft with the unique locations of each individual patient’s branch arteries […]
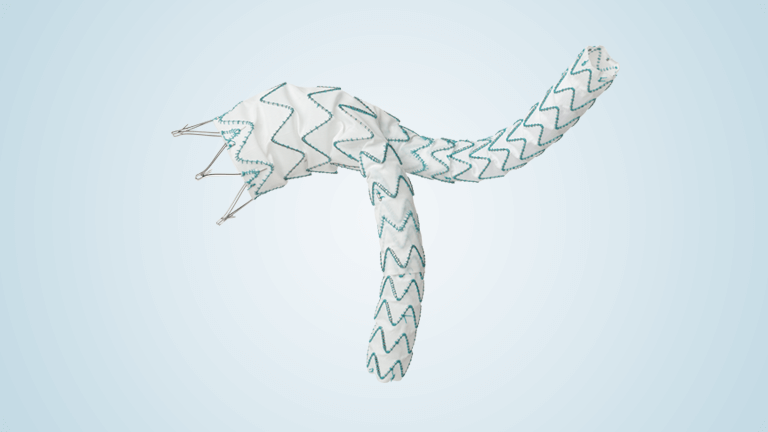
Terumo Aortic announces completion of enrolment of Relay®Pro US pivotal study
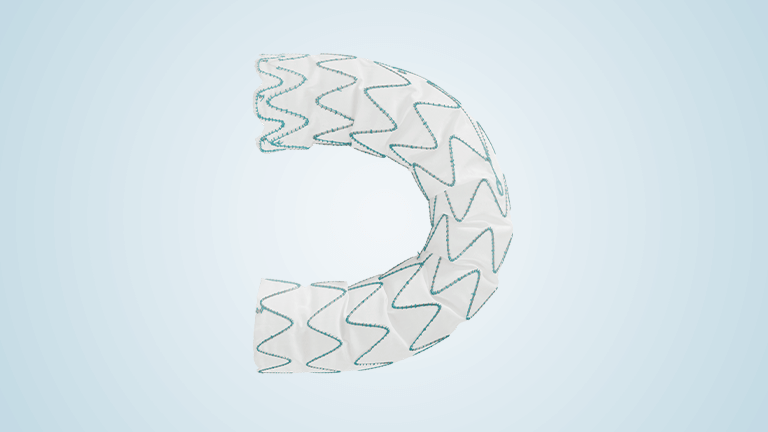
RelayPro is a low profile, next generation thoracic stent graft device designed to expand the treatment of thoracic endovascular aortic repair (TEVAR) to patients with smaller access vessels. RelayPro, having obtained CE Mark in 2018, utilises the same stent design, material and dual sheath technology already proven in Terumo Aortic’s Relay®Plus device, with the additional […]
Terumo Aortic announces launch of industry-first global endovascular registry

TiGER is a global, prospective, multi-arm, multi-centre registry evaluating more than 1,000 patients over a period of 5 years. The study will collect a broad range of clinical evidence on the company’s comprehensive thoracic and abdominal endovascular product portfolio. At the recent European Investigators’ Meeting, the TiGER Principal Investigator, Professor Vincent Riambau, Barcelona commented, “This […]
£33m investment in Scottish medical device factory

The investment has been approved for the major expansion of Vascutek’s headquarters and manufacturing facility at Inchinnan, Renfrewshire to help drive further growth for the global medical device company. Vascutek produces a wide portfolio of implants with a particular focus on treating patients with aortic disease. These implants have helped to save or improve the […]
Vascutek Wins 8th Queen’s Award for Innovation

VASCUTEK won the Queen’s Award for Innovation 2016 in April following the development of Thoraflex™ Hybrid, a surgical graft used to treat thoracic aortic disease, which affects the aorta, the largest artery in the body. The device, which is manufactured in Scotland, removes the need for a second major operation to treat the condition. It […]