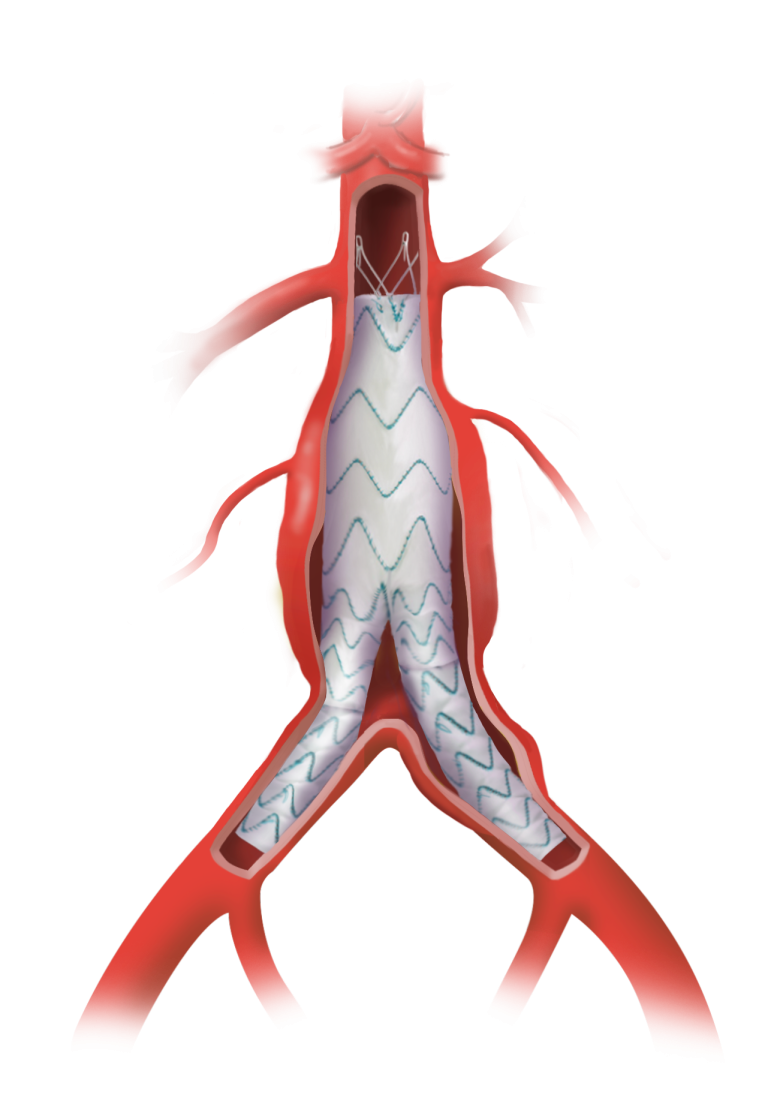
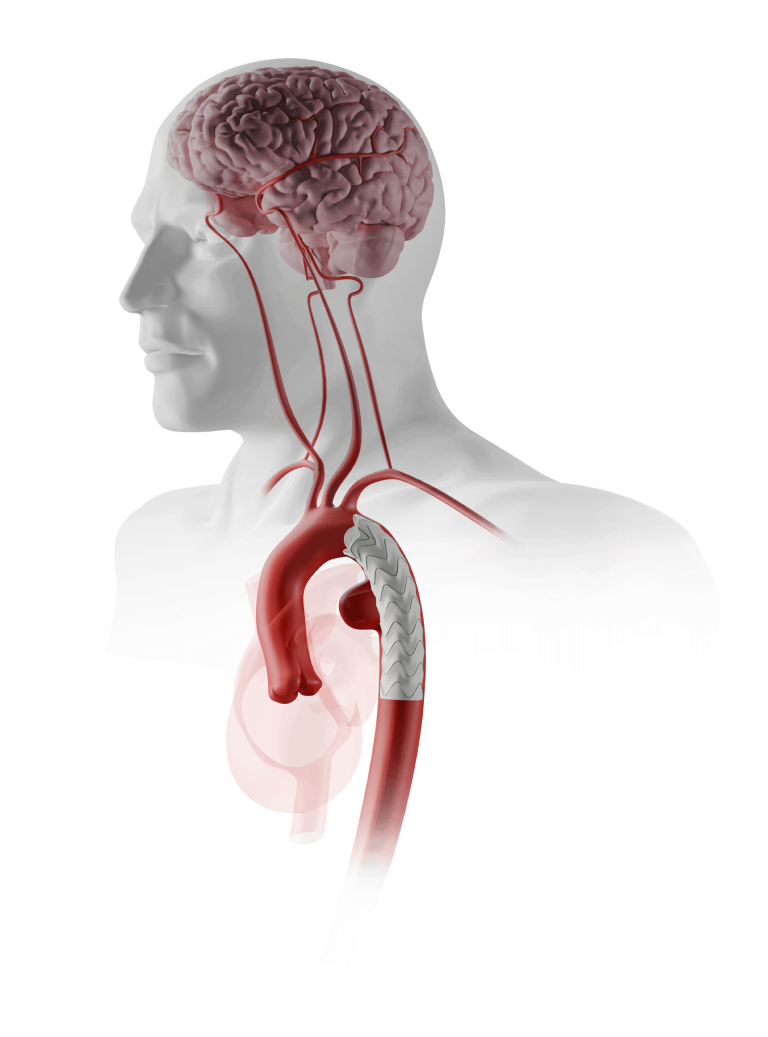
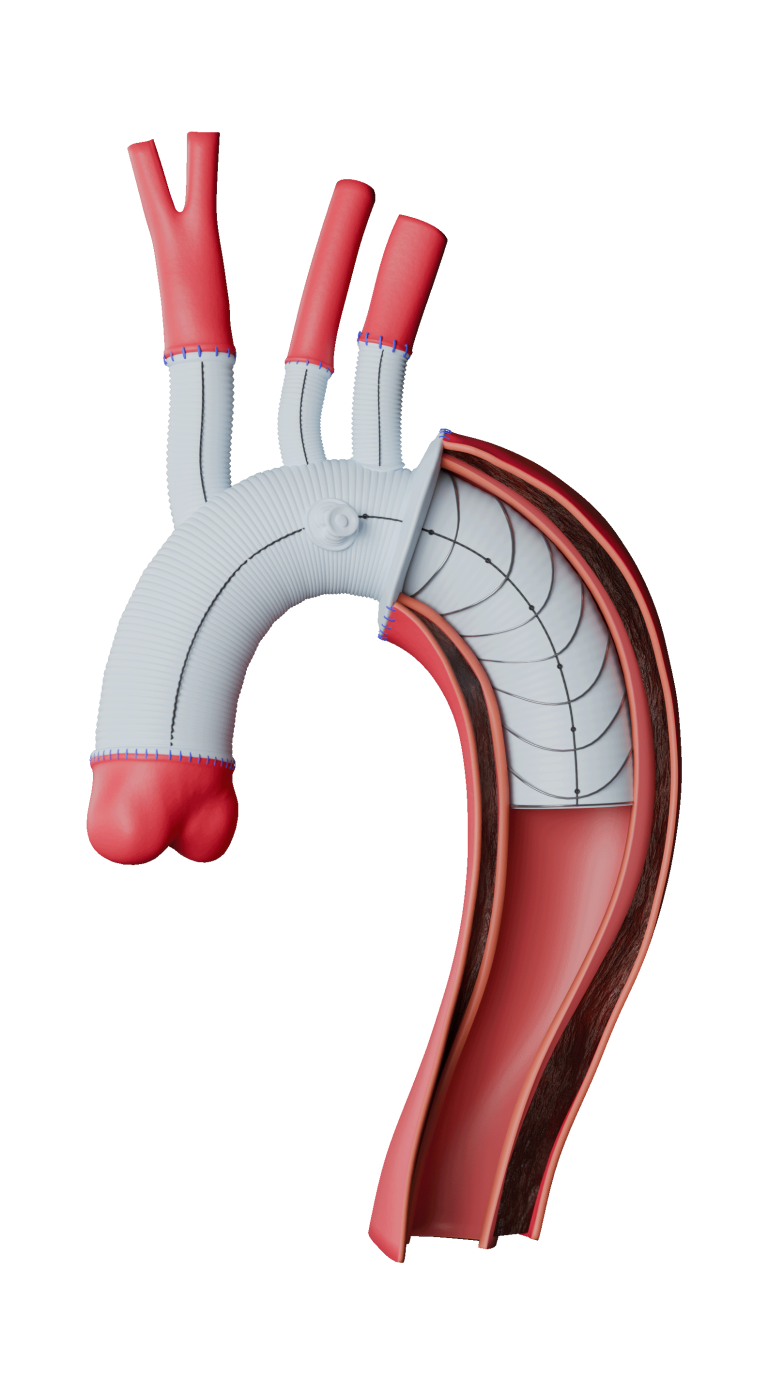
Caution: Custom made devices are not available in the USA
Custom Thoraflex™ Hybrid
Experience Optimised Intervention
Create bespoke solutions for your complex aortic arch repair cases. Adapt gold standard solutions to individual patient needs with the Thoraflex Hybrid Thoracic Aortic Stent Graft System (Custom made) – (Custom Thoraflex Hybrid).
Tailored Solutions Adapted for Patient Needs
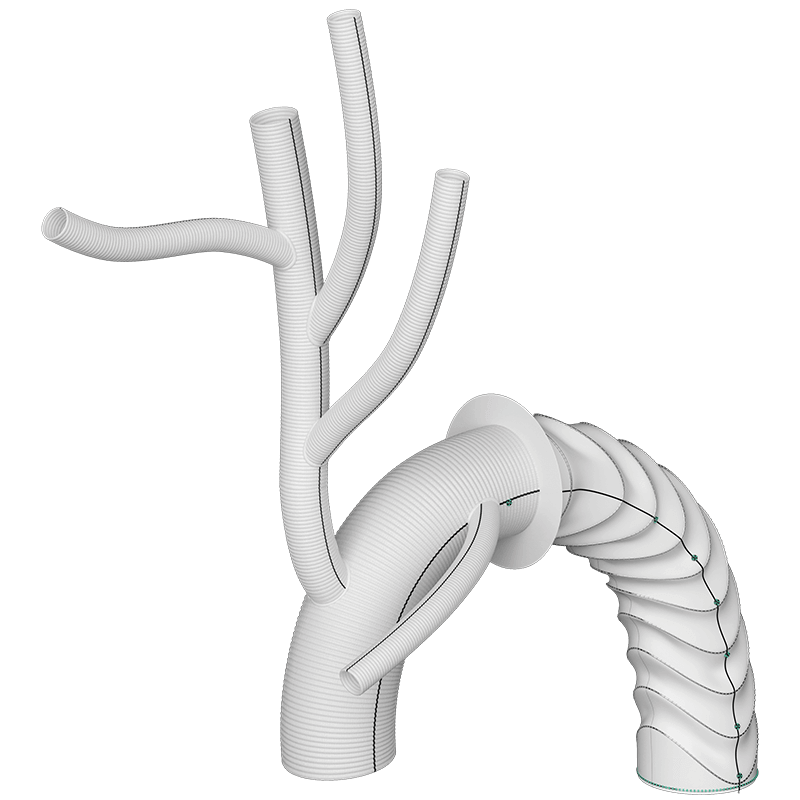
Arch Branches
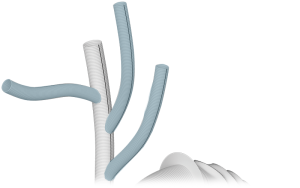
- 0-7 main branches (including bifurcate/side branch)*
- Adjusted angles, positioning and spacing*
- Diameters of 8/10/12/14/16/18mm*
- Lengths up to 150mm*
*Within Design Parameters
Side Branch
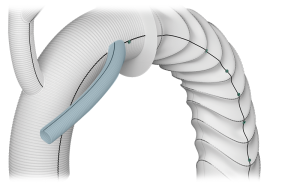
- Adjusted angle and orientation*
- Diameter 10mm
- Length 150mm
*Within Design Parameters
Radiopaque Markers
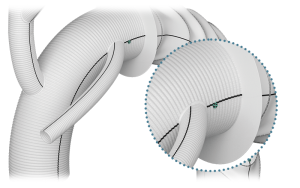
- Maximum of 2 (main body)
- Flexible positioning*
*Within Design Parameters
Stent Configurations

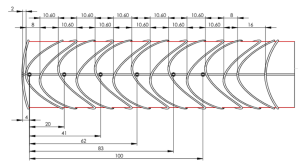
- Extraoffset 10° Mid-rings (increased radial force)
- Shorter stent (60mm minimum length)
- Combination of the two
A modification to the standard Thoraflex Hybrid design allowed improvement in operating times, complete and continuous cerebral trivascular perfusion, and correct positioning of the intrathoracic vessels.1
Paolo Masiello, M.D.
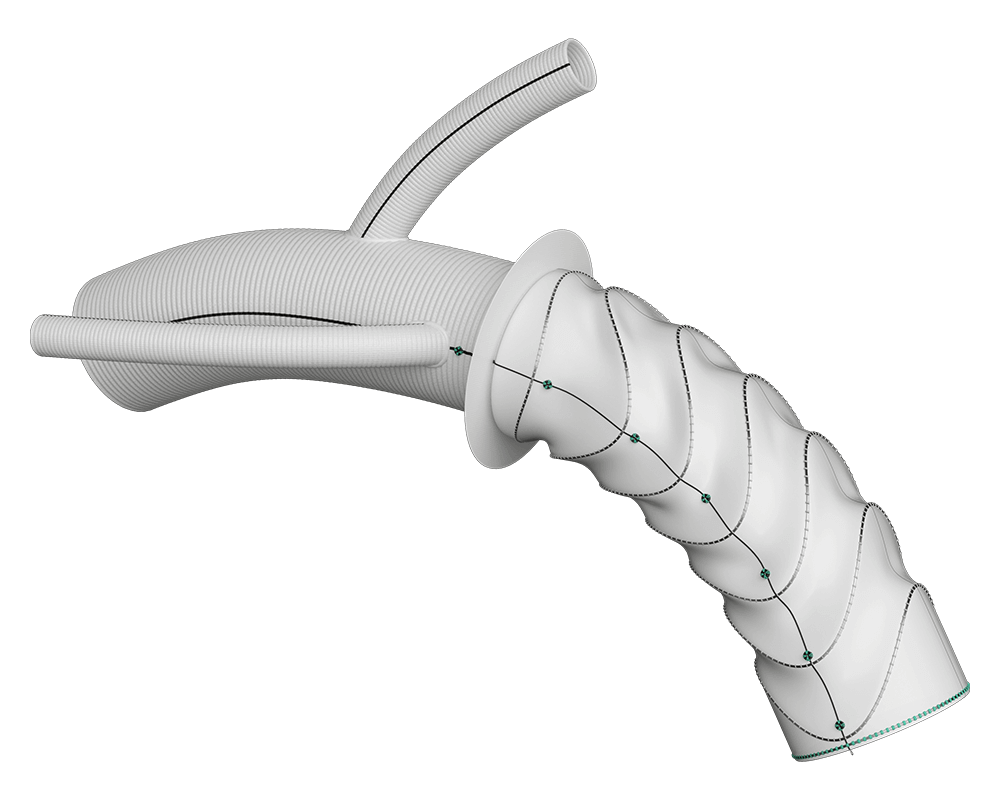
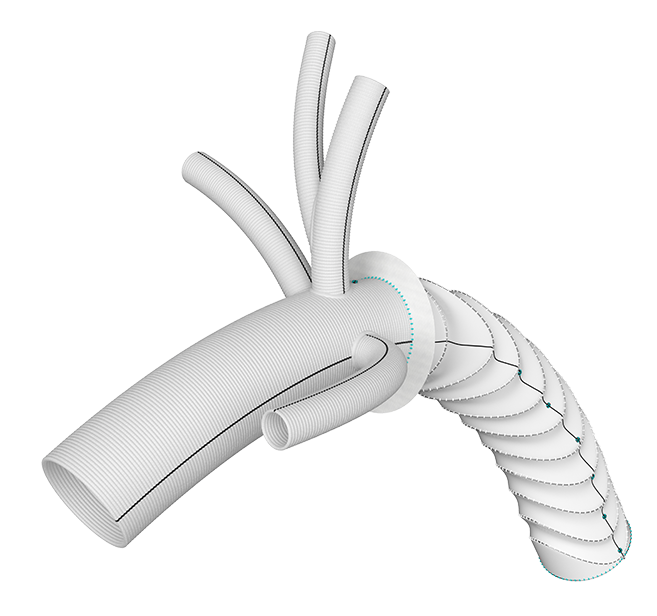
Secondary root and distal thoraco-abdominal re-interventions can be challenging after frozen elephant trunk. We obtained from the Terumo Aortic custom-made platform a Thoraflex graft with a modified disposition of the arch branches that facilitate secondary proximal and distal re-interventions. 2
Prof. Marco Di Eusanio
Customisation Options
| Feature | Variable | Parameters |
|---|---|---|
Body 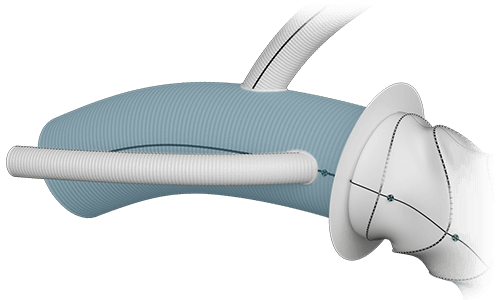 |
Length | ≤240mm |
| Diameter | 22-32mm | |
Arch Branches 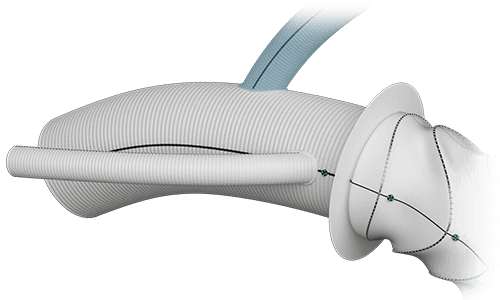 |
Diameter | 8/10/12/14/16/18mm |
| Angle | Variable | |
| Total | 7 | |
Perfusion Side Branch  |
Diameter | 10mm |
| Angle | Variable | |
Collar 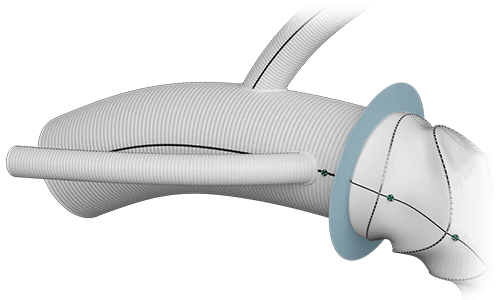 |
Width | 12mm |
Radiopaque Markers 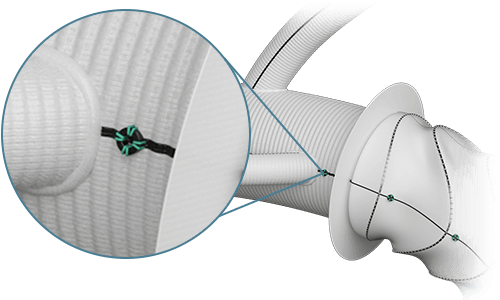 |
Total | 2 |
| Position | Variable |

We are committed to delivering the best solution to meet the needs of your patient and your practice.
Tailored Design
The Custom Thoraflex™ Hybrid programme provides you with options for patients with diverse aortic arch and distal anatomy that may require a customised approach.
Collaborative Service
Our team works hand in hand with you to deliver a customised solution for your patient.
Delivery: 6 weeks from design approval.
Caution: Custom Solutions are not available in the USA.
Downloads
- Rest of World
Downloads – Features & Benefits
References
Masiello P et al. 2021. A Modified frozen elephant trunk hybrid device to facilitate supra-aortic trunk anastomosis. Journal of Cardiac Surgery. 36:371–373
Di Eusanio et al. 2023. “T-Next: A new custom-made Thoraflex graft to simplify proximal and distal aortic reinterventions.” European Journal of Cardio-Thoracic Surgery, 63 (6), pp1-3.
Product Disclaimer
Custom made devices are specifically made in accordance with a written prescription of any person authorised by national law by virtue of that person’s professional qualifications; which gives (1) specific design characteristics provided under that person’s responsibility and (2) is intended for the sole use of a particular patient exclusively to meet their individual conditions and needs.
Custom made devices are not available in the US and availability is subject to local regulatory approval.
Instructions for Use
An IFU is provided with each custom device.
View the eIFU for more information on use, indications, contraindications, warnings/precautions and availability within your market.
Contact us
Click the button below to get in touch. For more updates, follow us on X and LinkedIn. You can also view our VuMedi channel.