This first implant was undertaken by Takao Ohki, MD, Chairman of the Department of Surgery, Professor and Chief of the Division of Vascular Surgery, Jikei University School of Medicine, Tokyo. Professor Ohki commented: “The procedure was very successful, the device performed well, and the patient is making a good recovery. The major concerns in endovascular surgery are stent migration and endoleaks; the TREO device provides both suprarenal and infrarenal fixation allowing for distribution of the stent-graft fixation in two different anatomical levels and is expected to result in superior long-term outcomes. In addition, the TREO device is sophisticated and very user friendly with a smooth, kink-resistant detachable sheath that definitely improves the accuracy and safety of the procedure.”
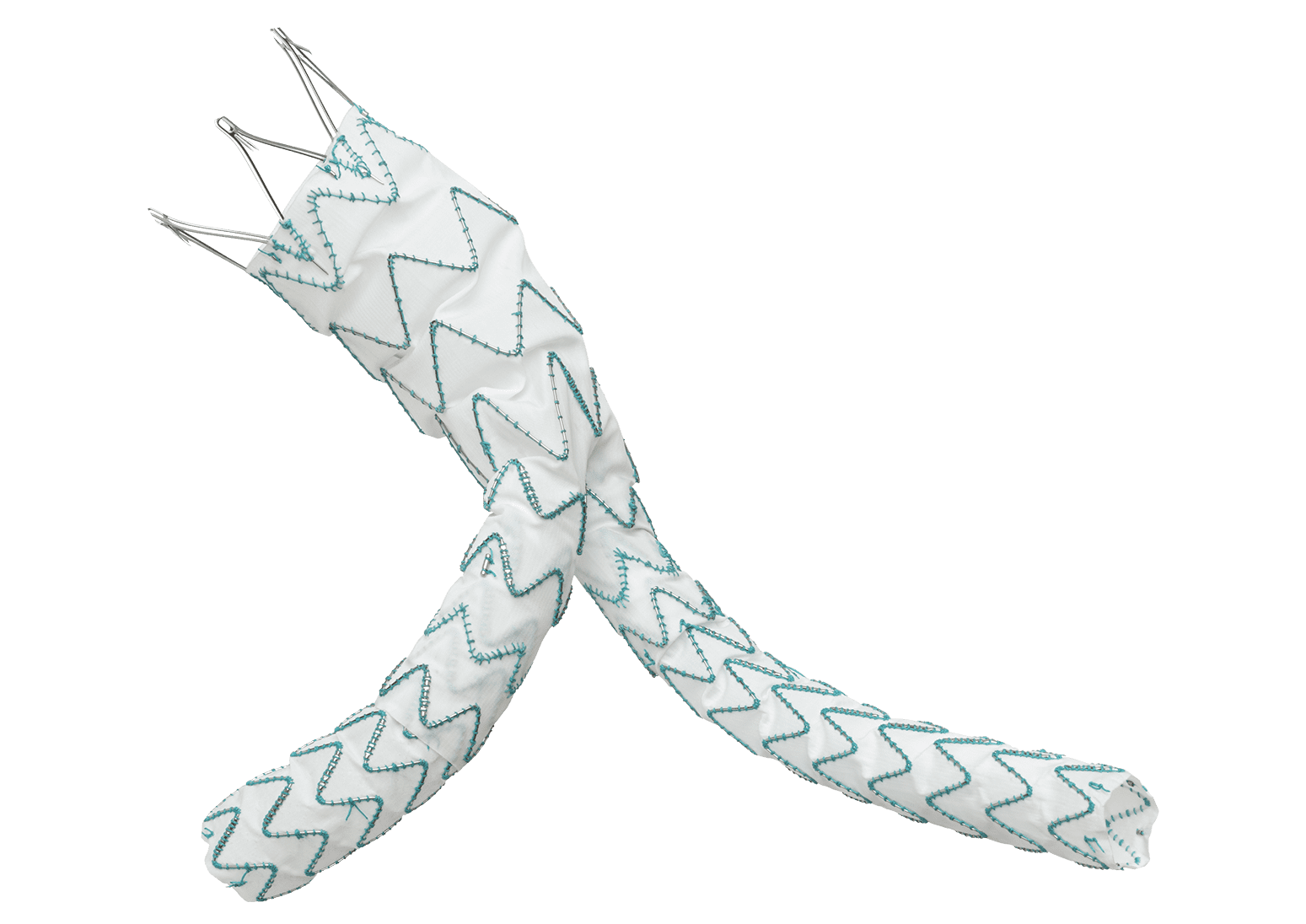
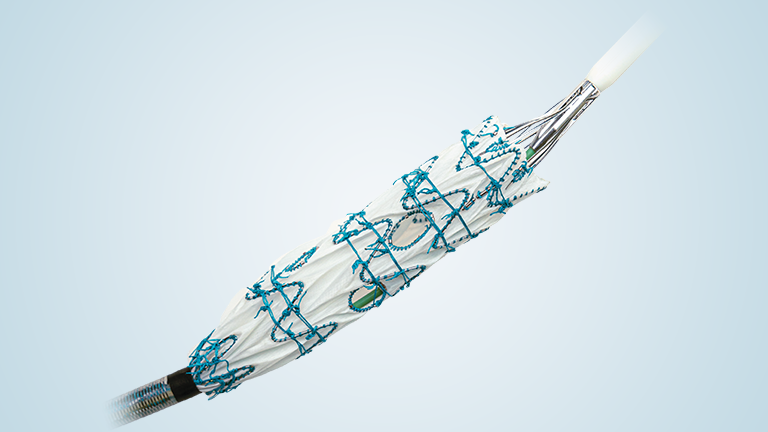
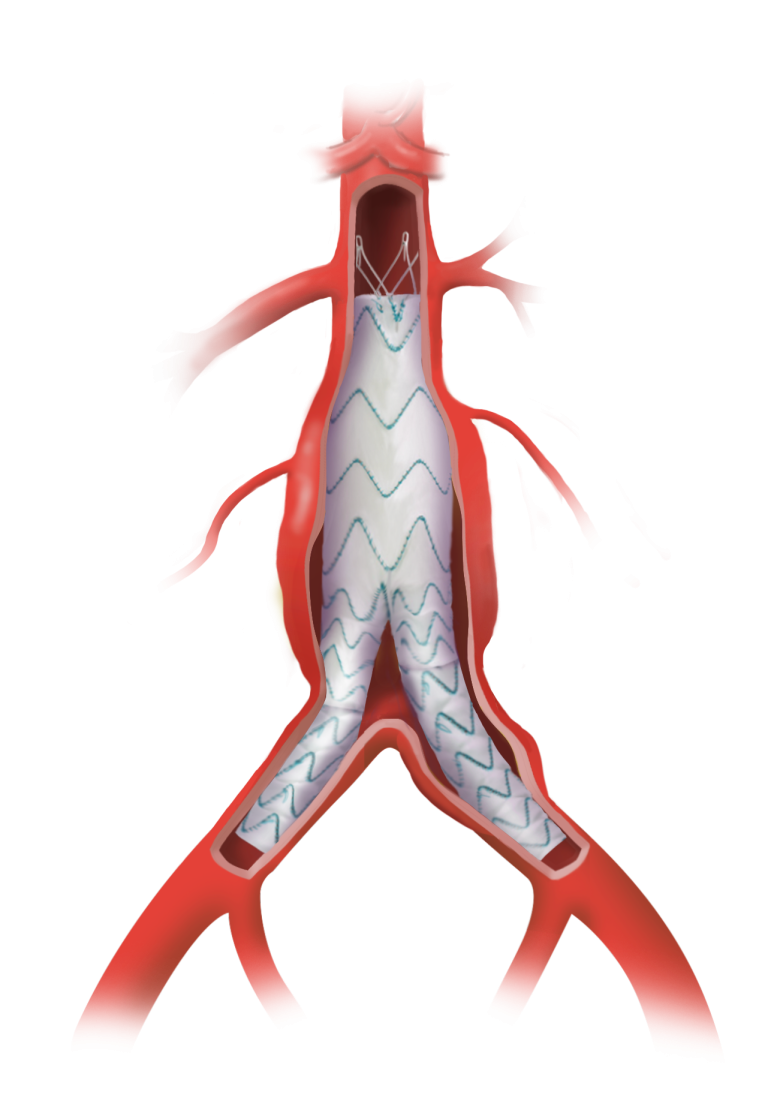
TREO is the only device commercially available for endovascular aneurysm repair (EVAR) with dual proximal fixation and lock stent technology offering physicians the next advancement in endovascular device solutions. It is a three-piece design featuring in situ limb adjustability and provides a wide range of aortic device configurations to specifically address the anatomy of each individual patient. TREO is designed for accurate, controlled and safe deployment; the exclusive proximal clasping mechanism and unique leave-behind sheath simplify the procedure as highlighted in recent clinical studies.
Kotaro Yoshida, General Manager at Terumo Aortic East Asia added: “This implant represents a significant milestone and demonstrates our commitment to delivering the most comprehensive portfolio in the aortic space. We look forward to continued expansion of our endovascular solutions in Japan.”
TREO received CE Mark approval in 2015 and FDA approval in 2020. This device is integral to Terumo Aortic’s innovative portfolio of surgical, endovascular and hybrid devices to treat every segment of the aorta.