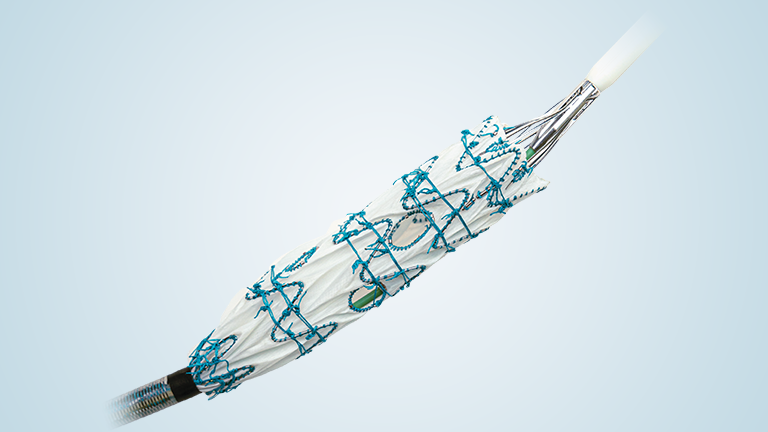
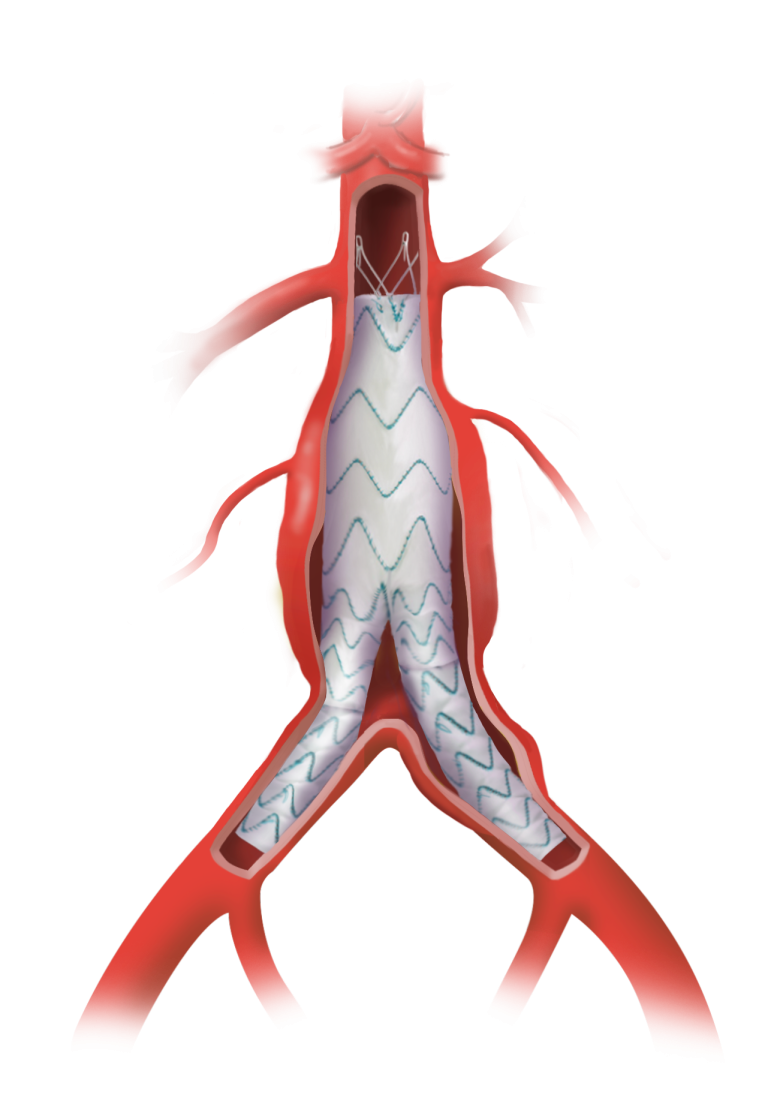
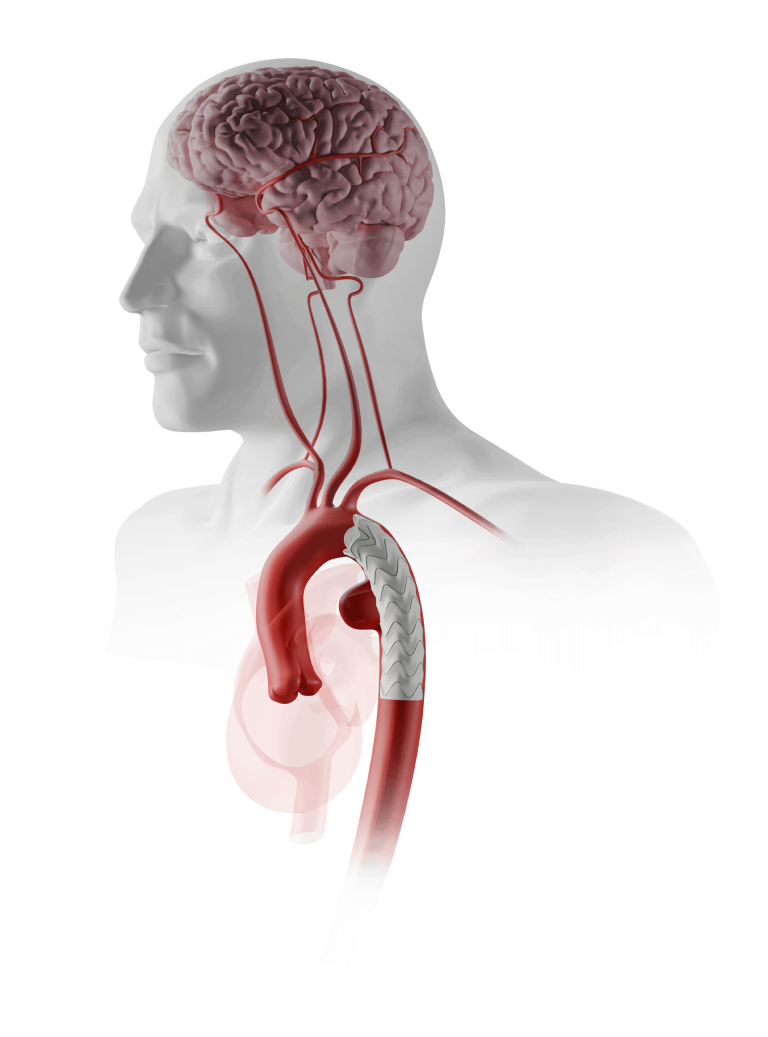
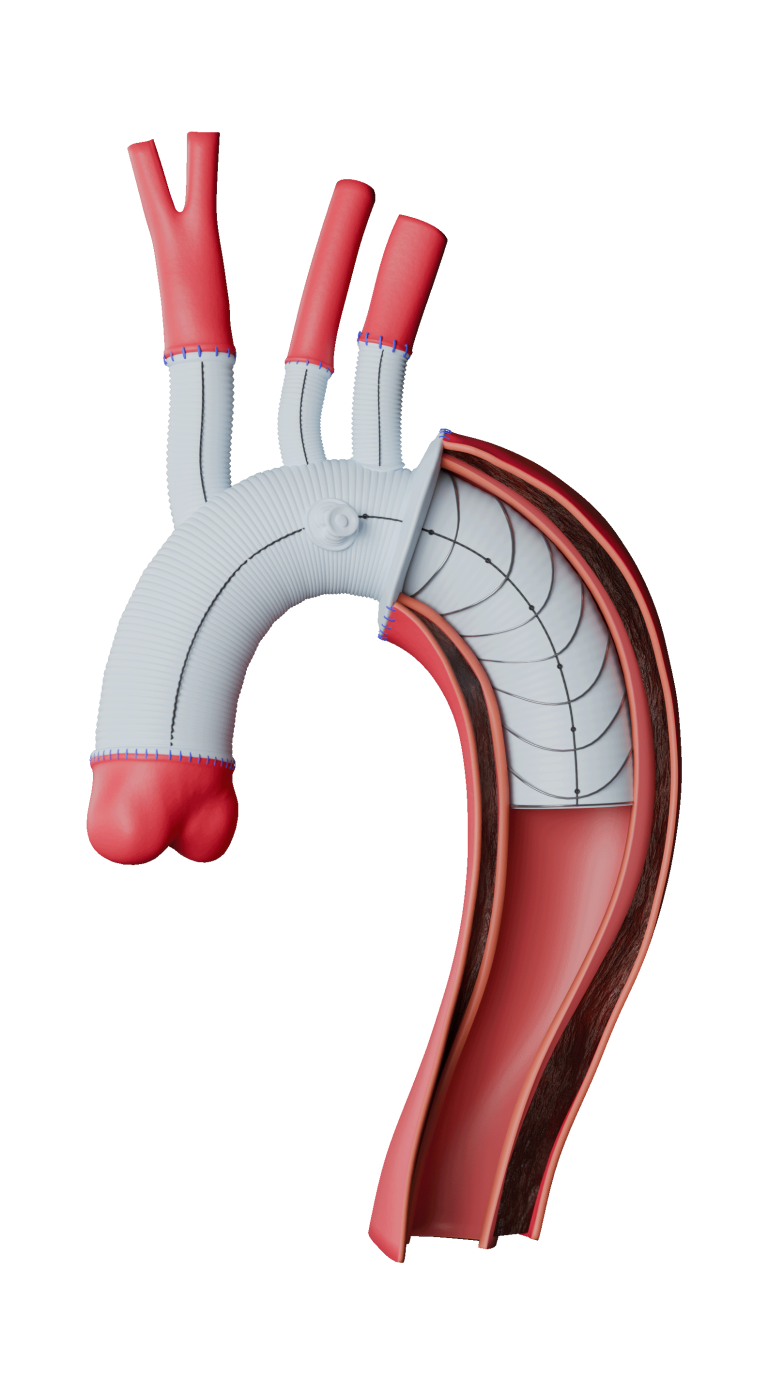
The device assists physicians in the expansion of the aorta when using TREO and RELAY stent-grafts in endovascular aortic repair.
Complementing our market leading product range of surgical and endovascular grafts, the Aortic Balloon is a low profile device with 10 F and offers exceptional inflation control and the broadest balloon diameter range, up to 50mm.
The first commercial use of the product was undertaken by Dr Siddharth Patel, Vascular Surgeon, Northside Vascular Surgery, Atlanta, GA during an implantation of a TREO abdominal stent-graft. Dr Patel commented: “The Aortic Balloon performed very well, with good inflation control, and rapid inflation and deflation. Also, its low profile gave me the ability to utilize the 13F leg extension detachable sheath of Treo for stent-graft remodelling without exchanging sheaths.”
Paul Kuznik, President of Terumo Aortic North America commented: “We are delighted to bring this low-profile balloon to the market and strengthen our credentials as a onestop shop for aortic solutions.”
This device is integral to Terumo Aortic’s innovative portfolio of surgical, endovascular and hybrid devices to treat every segment of the aorta.