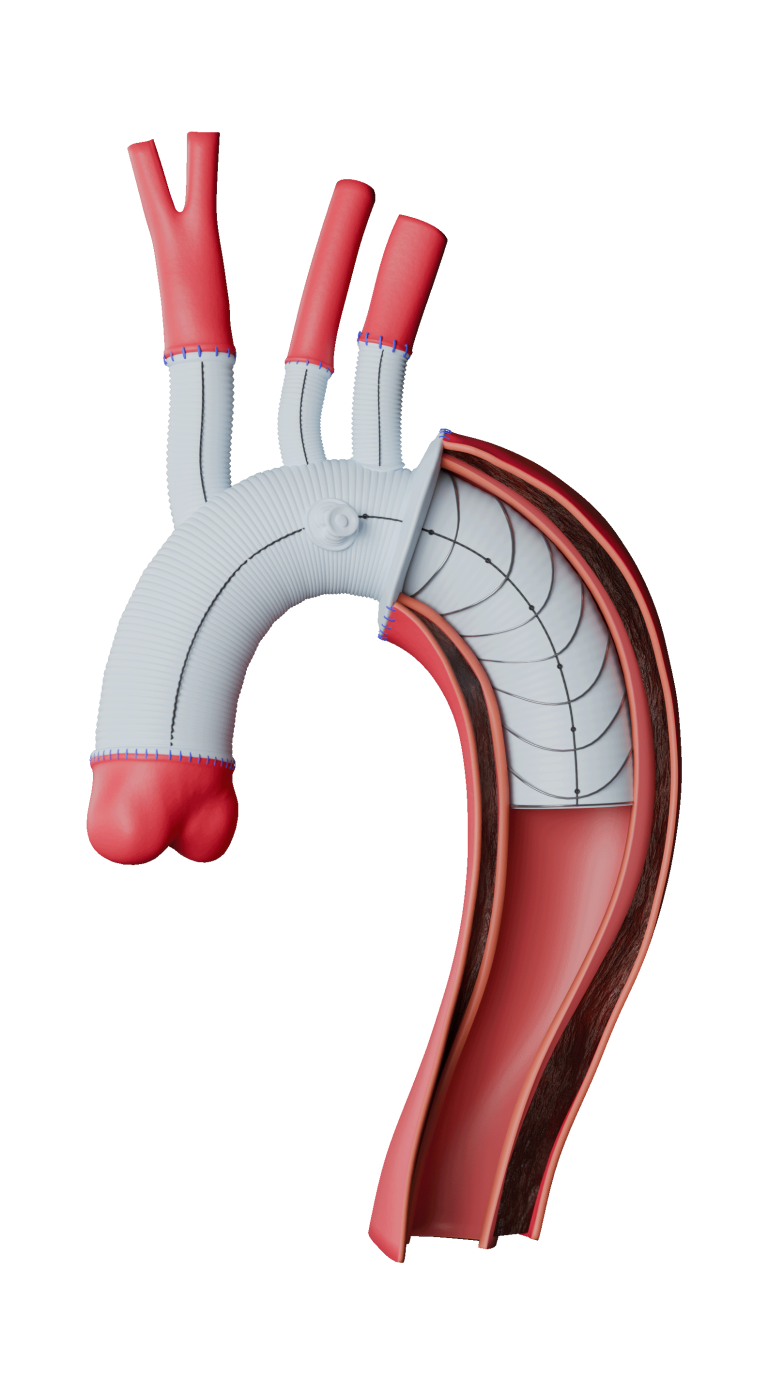
Caution: Custom made devices are not available in the USA.
Custom-Made Relay® Branch
Built to Accommodate
Custom-made Relay® Branch provides an effective solution for the treatment of aortic arch disease. 1
Addressing Challenges in the Aortic Arch
The Custom-made Relay® Branch provides an effective solution for the treatment of aortic arch disease. 1
Depending on the pathology and the targeted supra aortic vessel(s), this program can provide:
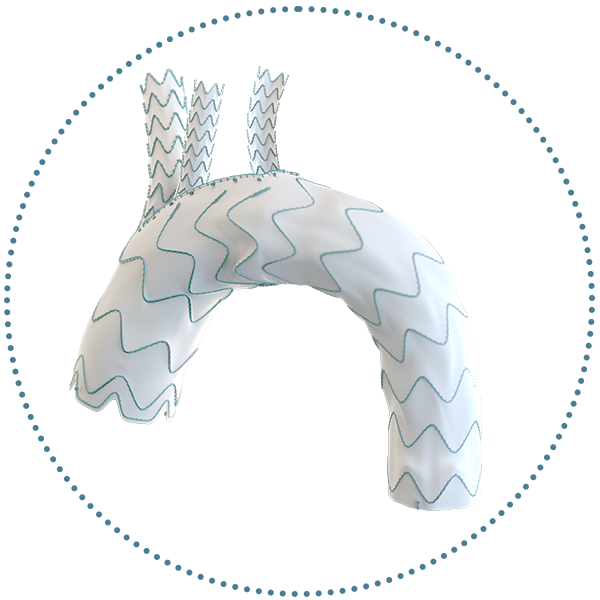
Triple Branch
- The complete endovascular solution for the repair of the aortic arch
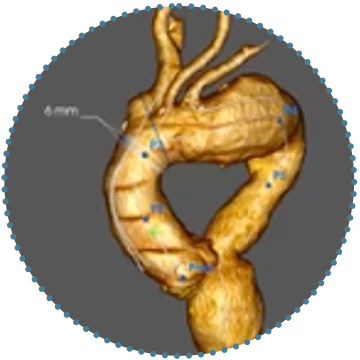
2
2

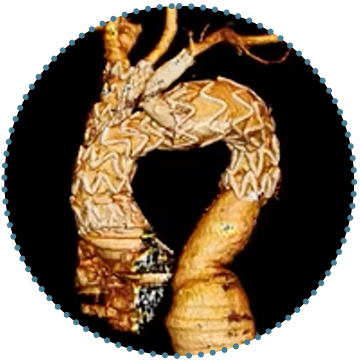
Double Branch
- Two tunnels to be deployed under the BCT and LCCA
3

3

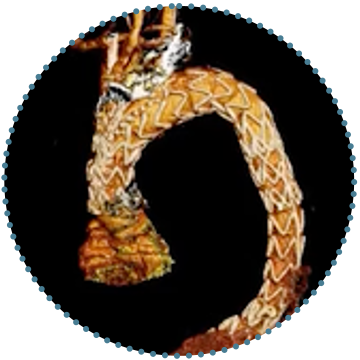
Single Branch
- One tunnel to be deployed under the LSA
4

4
The Single Branch Customisation may include a proximal scallop to proximally extend the landing zone
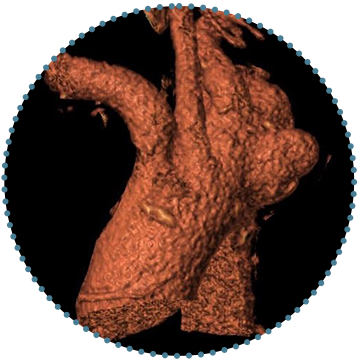
5
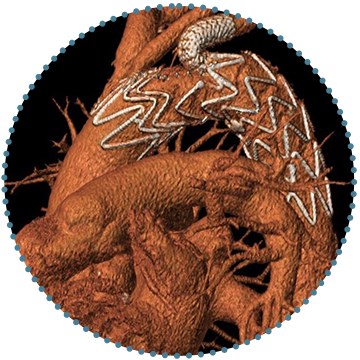
5
Addressing Challenges in the Aortic Arch
The Custom-made Relay® Branch builds upon the core features of the standard Relay®Pro platform, incorporating the same advanced technology and design principles, but with added customisation to address a specific patient need. The benefits of this platform include:

Graft
- Potential customisation options
- Wide window customisation
- Lock stent technology (where necessary)

Delivery System
- Dual Sheath Technology
- Pre-Curved Inner Catheter
- Support wires
- Asymmetrical Proximal Clasping
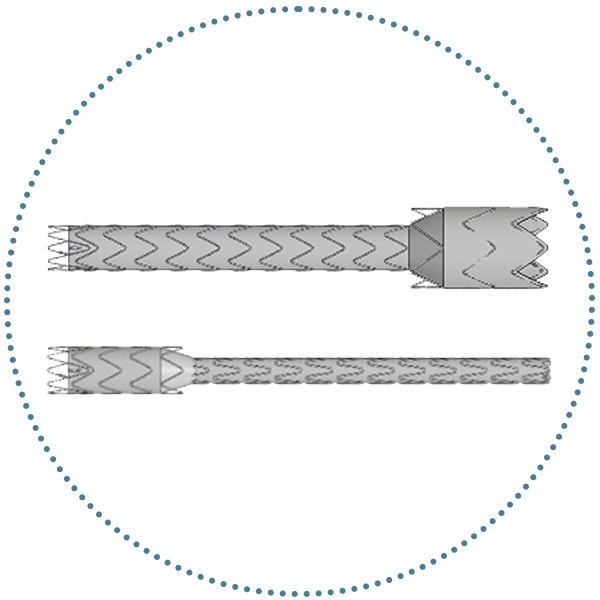
Branches
- TREO-based limb design
- Proximal clasping and short nose cone delivery system
- Controlled deployment
- 14 Fr O.D delivery system
Potential Customisation Options
Tailored Design for Every Need
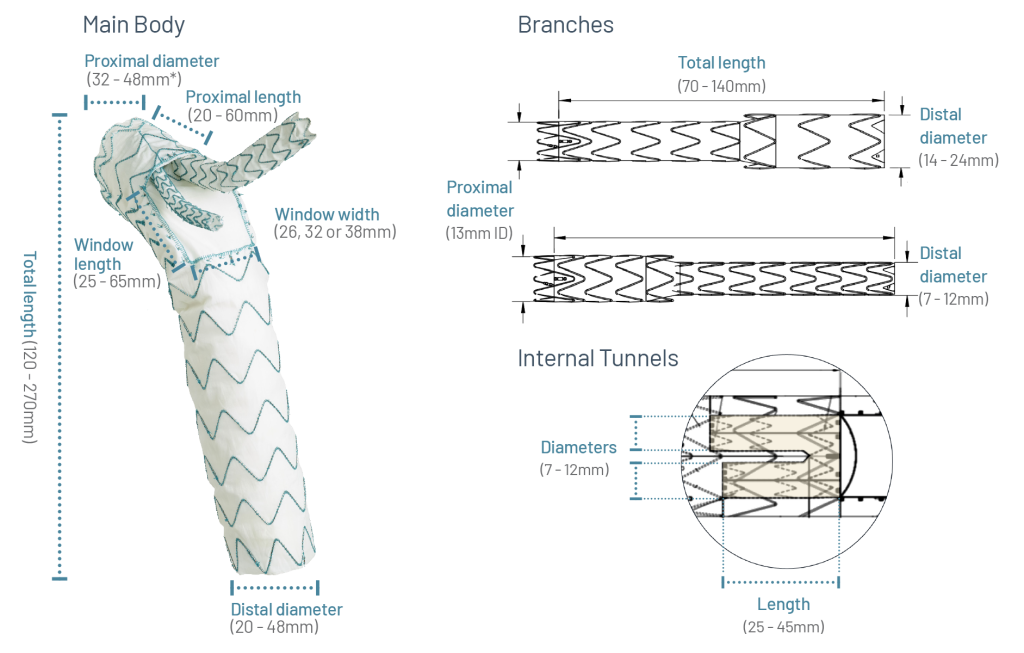
* The Relay Triple Branch and Relay Branch Proximal Scallop with Retrograde tunnel main bodies with 42mm or greater proximal diameter are loaded onto the RelayPlus delivery system.
Note: Measurements may vary depending on the specific configuration and the antegrade or retrograde position of the tunnels.
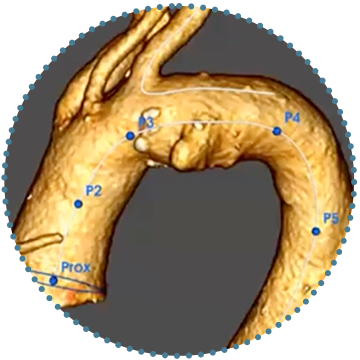
Wide Window Customisation
Designed for Easy Cannulation
- The cannulation window is designed to align to the outer curvature of the aorta to help simplify branch cannulation
±48min Mean Operative Time Including Cervical Bypassing6
Median Fluoroscopy Time7
"The large window [...] makes cannulation easy and fast while maintaining cerebral perfusion" 7
Ferrer et al.

Lock Stent Technology*
Designed to prevent disconnection
- Dull barbs facing towards lumen of the tunnel designed to prevent potential disconnection of the branches 7
Type III Endoleak 7
At 18-month mean follow up
0/20
Branch disconnection or migration 7
At 18-month mean follow up
"The presence of rounded barbs inside the tunnels (lock stent system) may also explain the absence of migration or disconnection phenomenon and subsequently of type III endoleak" 7
Ferrer et al.
* Lock stents are available upon request on every configuration and are limited to 12mm tunnel diameter. They are required when a TREO-based branch limb design is requested as a branch.
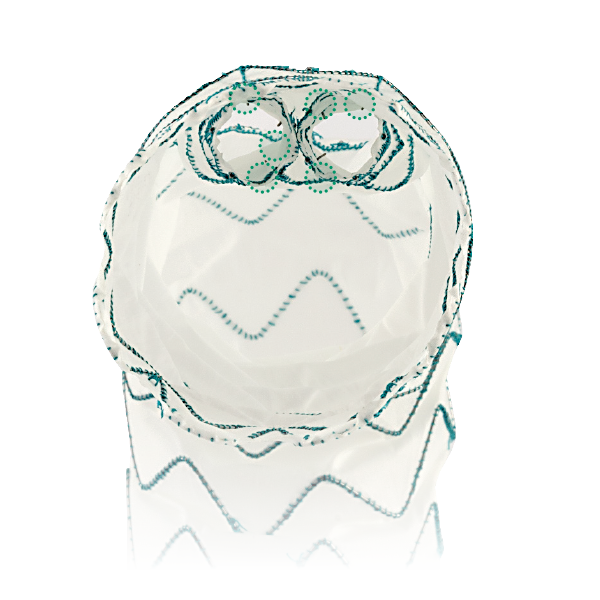
Dual Sheath Technology and Pre-Curved Nitinol Inner Catheter
Navigating the arch with care, designed for self-alignment
- Dual sheath delivery system, to facilitate the atraumatic advancement into zone 0
- Pre-curved nitinol inner catheter, to align the cannulation window to the outer curvature of the aorta
Stroke rate at 30-day follow-up 9
1/12
[...] a “self-righting” inner sheath that aligns the large branch cannulation window to the outer curve [...].8
McClure et al.
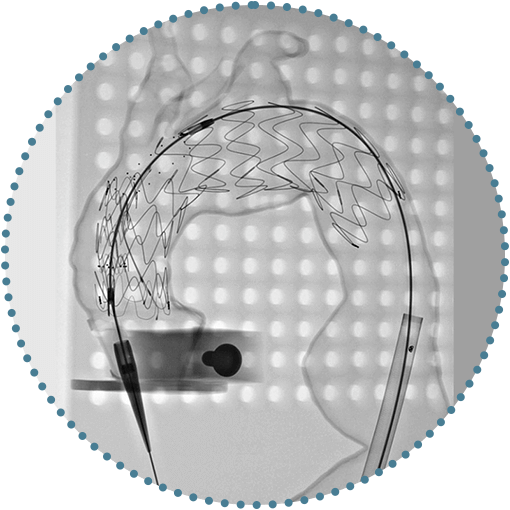
Benchtop Model
* Custom-made double branch devices are based on an evolutionary device Terumo Aortic has developed that was part of a limited, regulated IDE clinical study in the USA focused on advanced disease states that has enrolled 30 subjects. As with any endovascular surgical repair involving the aortic arch, implanting this type of multi-branch device may lead to a neurological event for the patient. Whilst indicating overall effectiveness so far, we are aware that a significant proportion of the subjects who have been implanted have experienced a neurological event. While these events have differed in severity (disabling and non-disabling) and timing (<24 hours to 4.5 years) post implantation and many have resolved, the overall rate is ~60%. Note that the subjects enrolled in the study had to meet specific inclusion/exclusion criteria, specifically those patients that are at very high risk or prohibitive risk for open surgical repair, which may not be applicable to an individual patient and custom-made device. After extensive and continued review of the events and potential contributing factors. mitigations have been implemented and Terumo Aortic, in conjunction with its physician advisory committee, continue to seek additional methods and technologies to further reduce the event rate. There is no further enrollment in this study.
Support Wires and Asymmetrical Proximal Clasping
Precise and Controlled Deployment
- Two support wires guide the inferior portion toward the inner aortic wall, keeping it aligned with the
landing zone, minimising the risk of retroflex - Two clasped stent apices, both located on the outer curve, allow for precise and controlled
deployment
"The presence of pared “driving-wires” (support wires) allows for a precise proximal landing in Zone 0 and for progressive apposition of the proximal stent-graft segment against the aortic wall." 10
Riambau et al.
Early Type Ia Endoleak 11
1/43
Technical Success 9
12/12
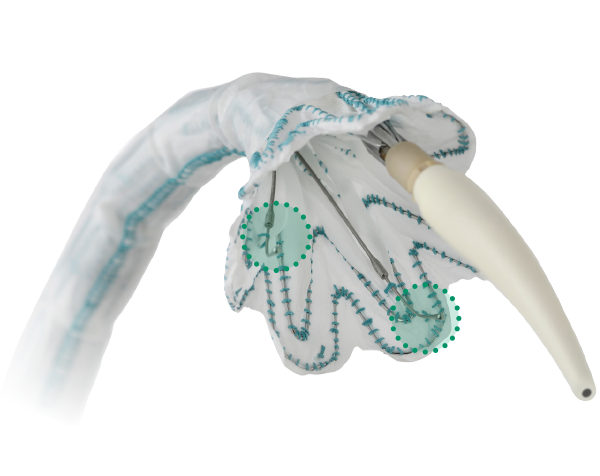
Branches
- TREO-based Design
- Proximal Clasping and Short Nose Cone delivery system
- 14 Fr O.D. with 49cm long detachable sheath delivery system
- “Mechanical advantage” featured in the delivery system for controlled deployment
"The branches are customized versions of the iliac limbs used with the Treo abdominal endovascular stent graft system" 1
Van der Weijde et al.

Delivery System

Proximal Clasping
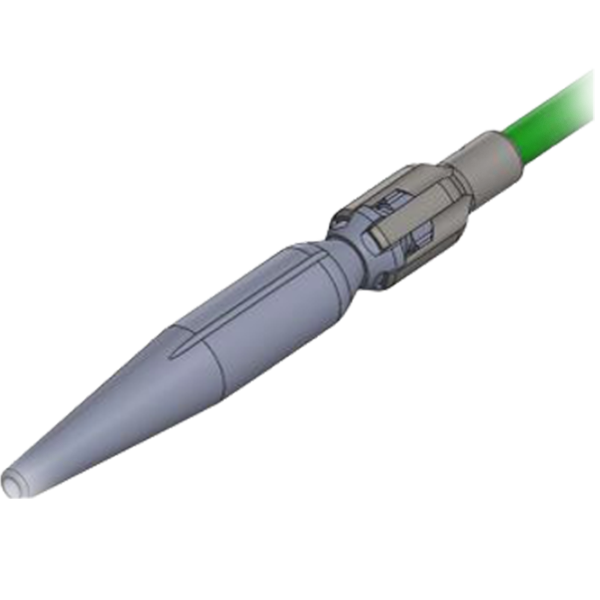
Short Tip

We are committed to delivering the best solution to meet the needs of your patient and your practice.
Tailored Design
The Custom Solutions programme provides you with options for patients that may require a custom approach.
Collaborative Service
Our team works hand in hand with you to deliver a customised solution for your patient.
Delivery: 6 weeks from design approval.
Caution: Custom Solutions are not available in the USA.
Downloads
Downloads – Features & Benefits
- Rest of World
References
Van der Weijde et al. (2019) – ‘Total Endovascular Repair of the Aortic Arch: Initial Experience in the Netherlands’, The Annals of Thoracic Surgery.
Case images courtesy of Prof. Piotr Szopinski, Institute of Hematology and Transfusion Medicine. Warsaw, https://www.vumedi.com/video/future-of-tevar-the-evolution-from-single-to-triplebranch- technology/?list=30f43900-2c2b-4624-830e-46dac385494e
Case images courtesy of Prof. Piotr Szopinski, Institute of Hematology and Transfusion Medicine. Warsaw https://www.vumedi.com/video/relayr-double-and-triple-branch-case-overview/
Case images courtesy of Dr. Martin Funovics, Cardiovascular and Interventional Radiology Medical University of Vienna, https://www.vumedi.com/video/future-of-tevar-the-evolution-fromsingle- to-triple-branch-technology/?list=30f43900-2c2b-4624-830e-46dac385494e
Case images courtesy of Dr. Florian Elger, UniversitätSmedizin Göttingen
Kudo et al. (2020) – Early and midterm results of thoracic endovascular aortic repair using a branched endograft for aortic arch pathologies: A retrospective single-center study, JTCVS Techniques
Ferrer et al. (2019) – iTalian RegIstry of doUble inner branch stent graft for arch PatHology (the TRIUmPH Registry) Journal of Vascular Surgery
McClure et al. (2021) – Zone 0 Aortic Arch Reconstruction Using the RelayBranch Thoracic Stent Graft CJC Open
Iglesias Iglesias et al. (2023) – An early single-center experience with the Relay double inner-branch arch endograft, Journal of Thoracic Disease
Riambau et al. (2015) – Application of the Bolton Relay Device for Thoracic Endografting In or Near the Aortic Arch, AORTA
Czerny et al. (2021) – Results of endovascular aortic arch repair using the Relay Branch System, European Journal of Cardio-Thoracic Surgery
Product Disclaimer
Custom made devices are specifically made in accordance with a written prescription of any person authorised by national law by virtue of that person’s professional qualifications; which gives (1) specific design characteristics provided under that person’s responsibility and (2) is intended for the sole use of a particular patient exclusively to meet their individual conditions and needs.
Custom made devices are not available in the US and availability is subject to local regulatory approval.
As with any endovascular repair involving the aortic arch, implanting this type of device may lead to a neurological event and the associated risks should be thoroughly considered.
Instructions for Use
View Custom-Made device eIFU for more information on use, indications and warnings/precautions.
Contact us
Click the button below to get in touch. For more updates, follow us on X and LinkedIn. You can also view our VuMedi channel.