Terumo Aortic announces PMDA Approval and first commercial implant of TREO endovascular device in Japan
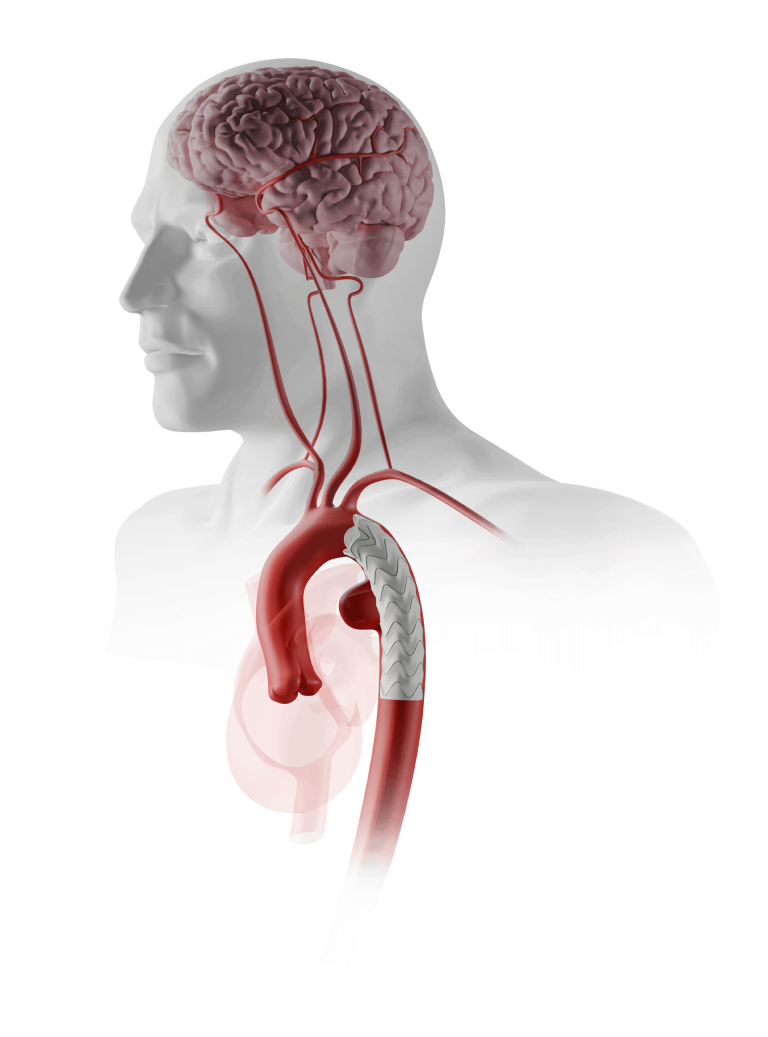
This first implant was undertaken by Takao Ohki, MD, Chairman of the Department of Surgery, Professor and Chief of the Division of Vascular Surgery, Jikei University School of Medicine, Tokyo. Professor Ohki commented: “The procedure was very successful, the device performed well, and the patient is making a good recovery. The major concerns in endovascular […]
Terumo Aortic announces publication of the primary endpoint results from the TREO Pivotal Study

Following recent approval by the US Food and Drug Administration (FDA) of the TREO® Abdominal Aortic Stent-Graft System for the treatment of patients with abdominal aortic aneurysms (AAA), Terumo Aortic announced today the publication of the primary endpoint results from the investigational device exemption (IDE) pivotal study in the Journal of Vascular Surgery.
Terumo Aortic announces US FDA Approval for TREO endovascular device

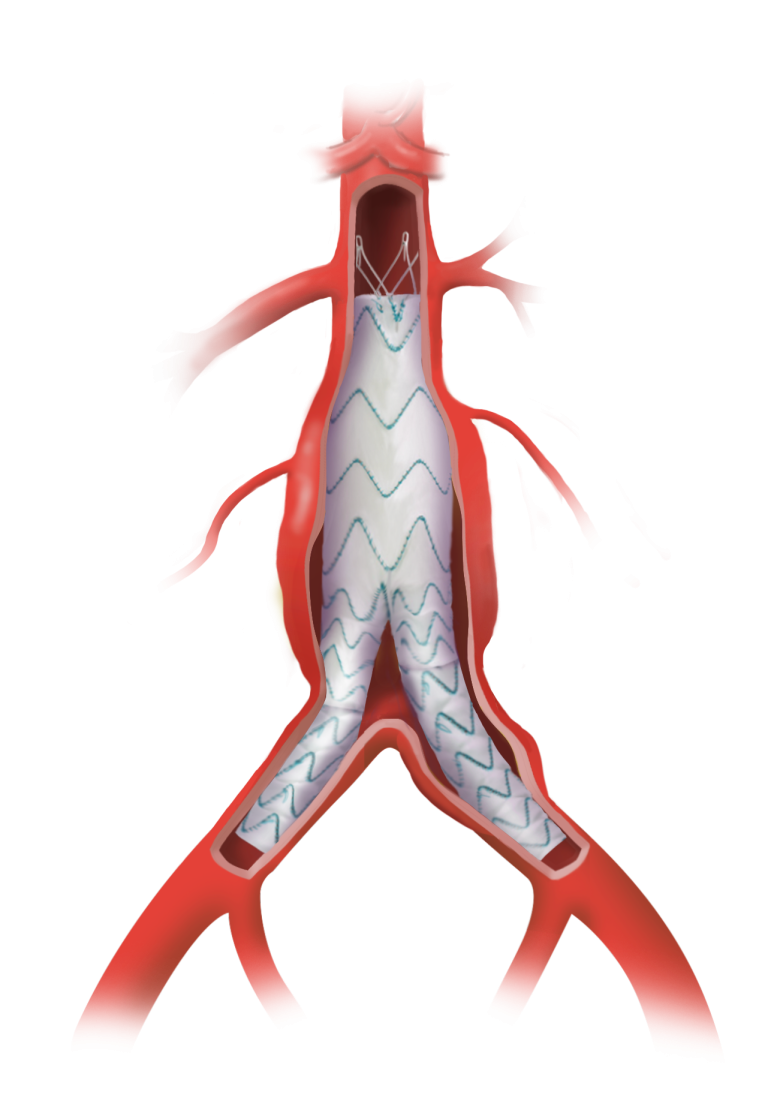
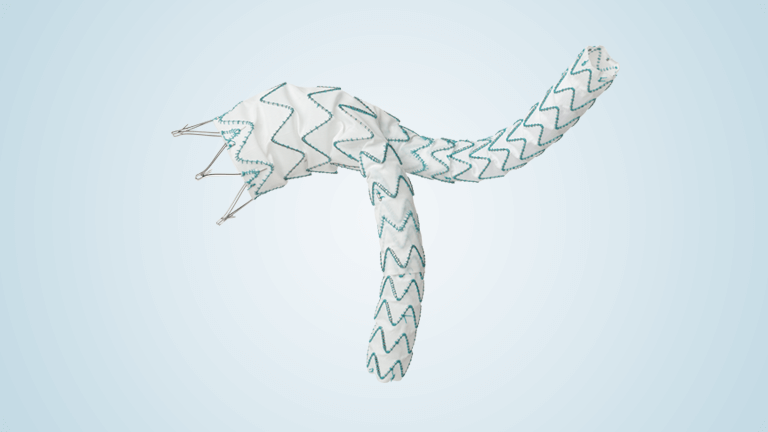
TREO is the only device commercially available for endovascular aneurysm repair (EVAR) with dual proximal fixation and lock stent technology offering physicians the next advancement in endovascular device solutions. It is a three-piece design featuring in situ limb adjustability and provides a wide range of aortic device configurations to specifically address the anatomy of each […]
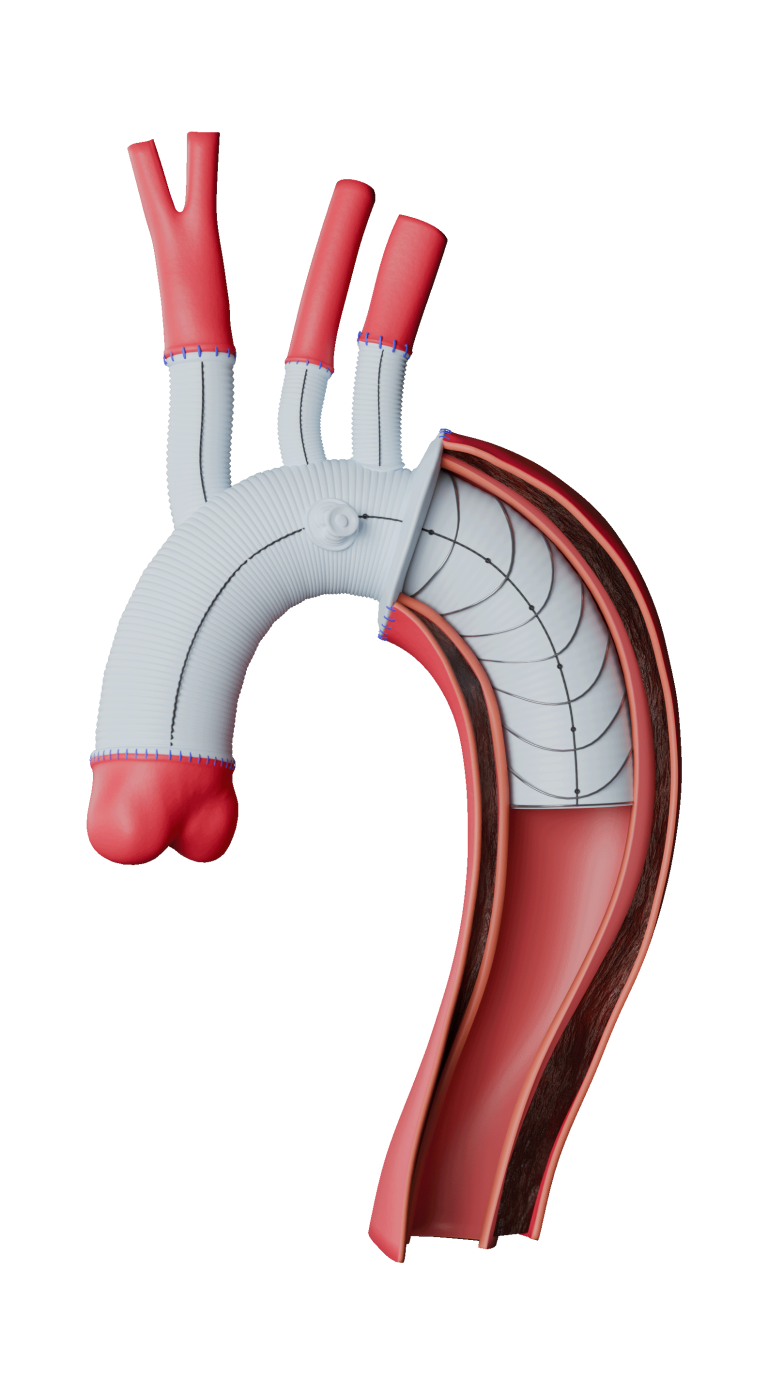
Terumo Aortic Announces US FDA Breakthrough Device designation for Thoraflex Hybrid device
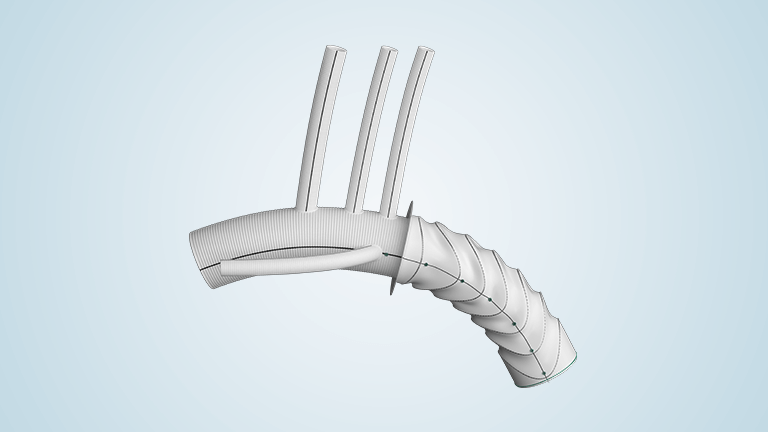
The purpose of the FDA’s Breakthrough Device Designation program is to fast-track the regulatory review process for certain medical technologies and device-led combination products that satisfy certain criteria; these include providing a more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions. The aim of the program is to provide patients and […]