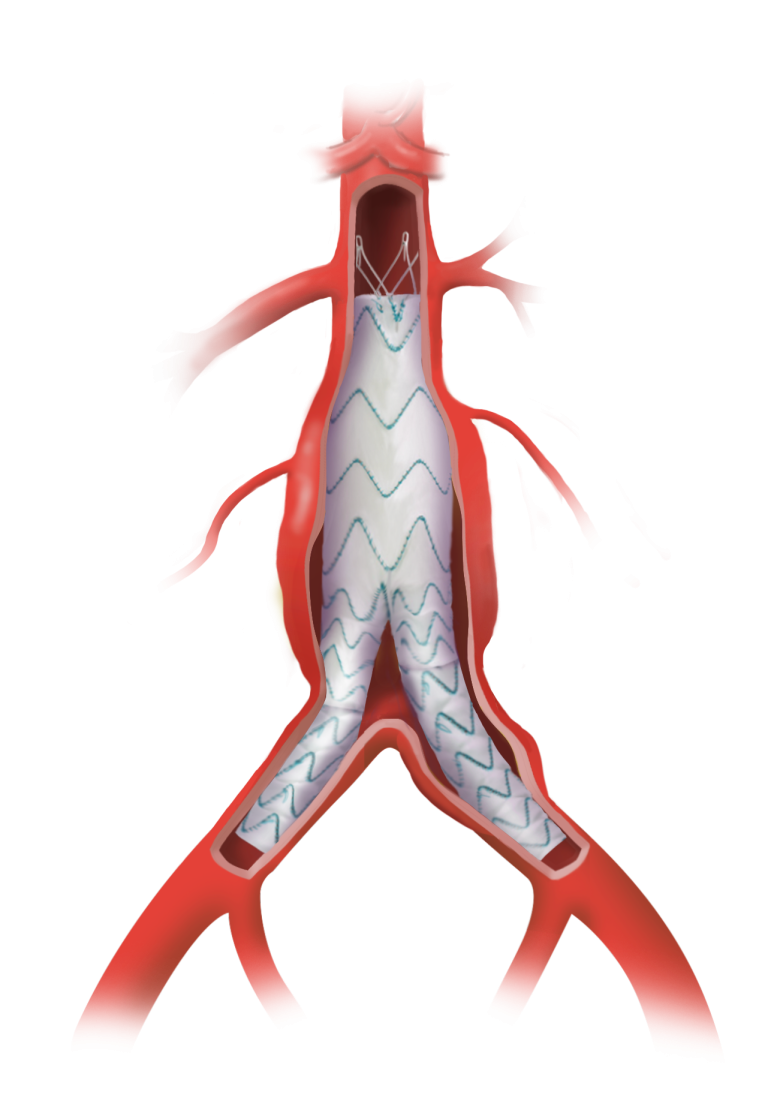
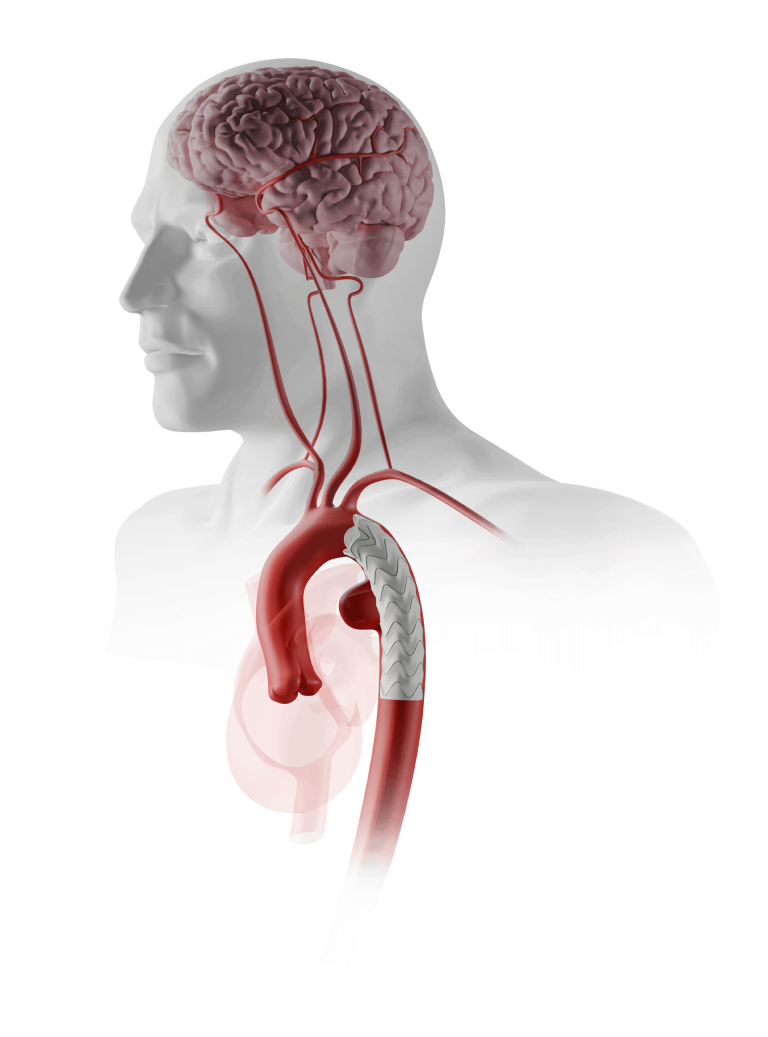
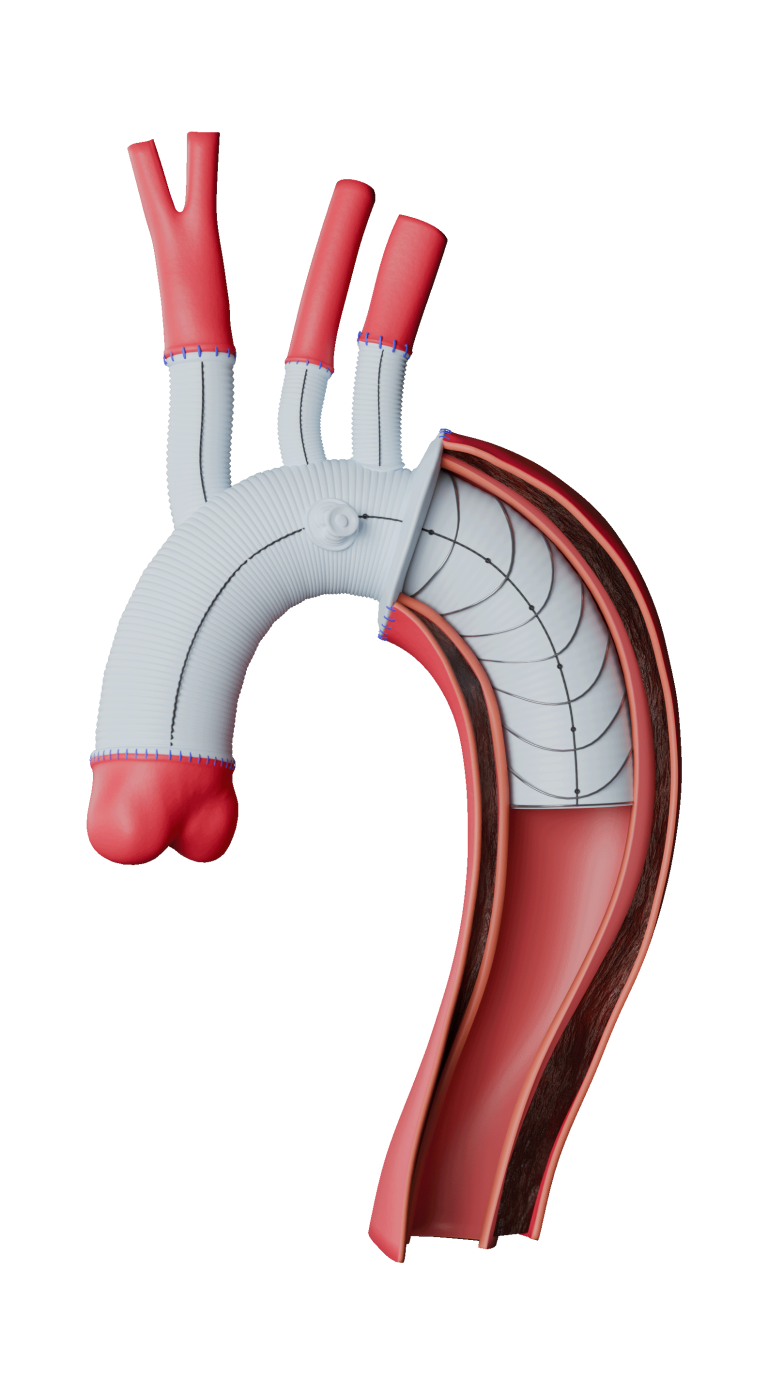
Caution: Custom made devices are not available in the USA.
Custom Fenestrated TREO®
Advancing Fenestrated Horizons
The Fenestrated TREO platform, based on the TREO® Abdominal Stent-Graft System, is a simple trimodular system.
The All-Encompassing Next Generation Fenestrated Endograft.
The Fenestrated TREO platform, based on the TREO Abdominal Stent-Graft System, is a simple trimodular system.

Reliable staged expansion for a controlled deployment
-
Circumferential suture wraps
-
Modified distal clasping to improve torque control
-
Ability to cannulate from above and below
-
Ability to reposition graft while partially deployed
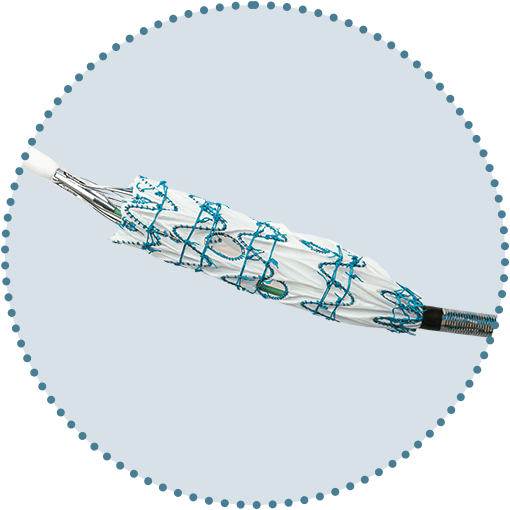
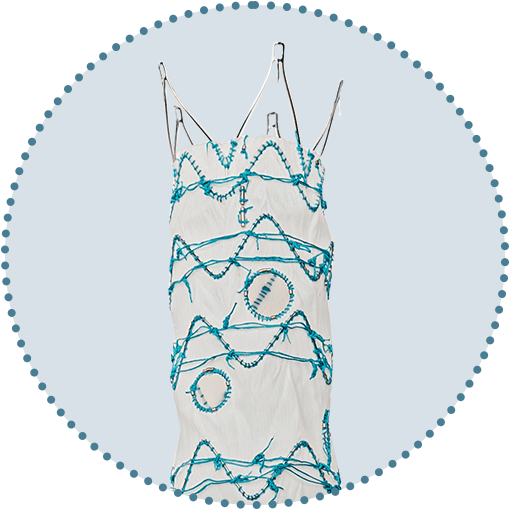
Freedom to position fenestrations
Up to 6 fenestrations
Freedom to position stents and fenestrations as required
Circumferential radiopaque marker for improved visualisation and alignment accuracy
Ability to reposition graft while partially deployed
Main body can allow up to 40mm of free space between springs for fenestration placement
Fenestration sizes up to 12mm
Optimised stent configuration
-
Fully configurable support positioning for optimal fenestration placement
-
Additional sealing stents can be added to provide a longer proximal sealing zone
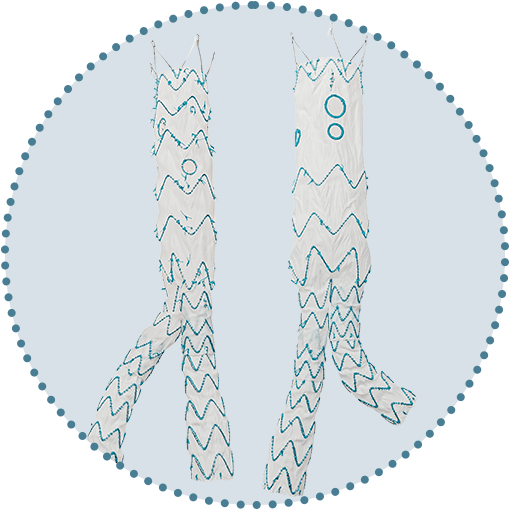

Ability to cannulate from above
Circumferential radiopaque marker for improved visualisation and alignment accuracy


Trusted low profile device
-
Reducing ties mechanism allows graft repositioning until completely opened
-
60cm low profile sheath with hydrophilic coating and flexible tip for easier navigation
-
The clasp mechanism keeps control of deployment and allows precise placement with cranial and caudal adjustment before the bare stent is released
-
The mechanical deployment provides controlled and stable stent-graft deployment
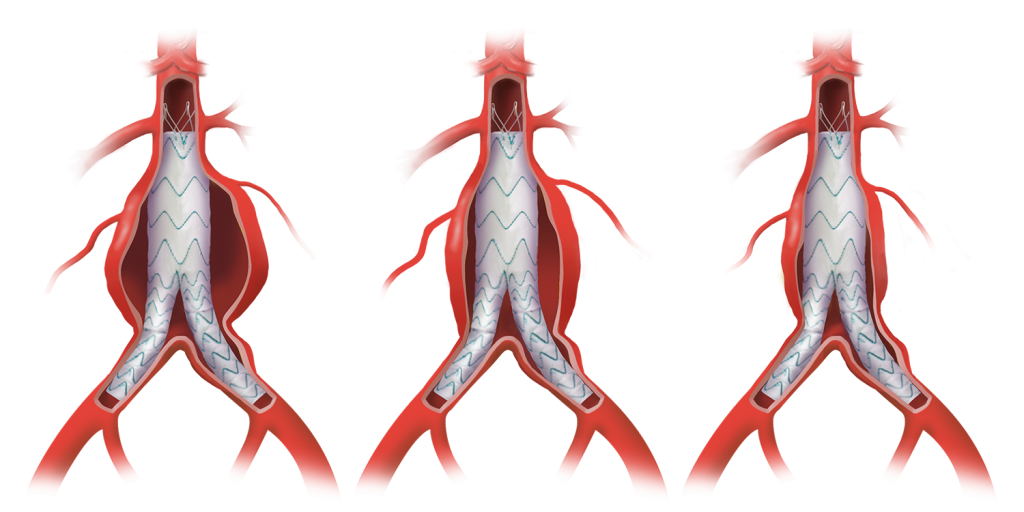
TREO®
Repair. Remodel. Results.
Learn how the TREO® abdominal stent-graft system supports EVAR success with sac regression, and aortic remodelling for improved outcomes.

We are committed to delivering the best solution to meet the needs of your patient and your practice.
Tailored Design
The Custom Solutions programme provides you with options for patients that may require a custom approach.
Collaborative Service
Our team works hand in hand with you to deliver a customised solution for your patient.
Delivery: 6 weeks from design approval.
Caution: Custom Solutions are not available in the USA.
Downloads
- Rest of World
Downloads – Features & Benefits
- Rest of World
References
Data on File
Product Disclaimer
Custom made devices are specifically made in accordance with a written prescription of any person authorised by national law by virtue of that person’s professional qualifications; which gives (1) specific design characteristics provided under that person’s responsibility and (2) is intended for the sole use of a particular patient exclusively to meet their individual conditions and needs.
Custom made devices are not available in the US and availability is subject to local regulatory approval.
Instructions for Use
An IFU is provided with each custom device.
View the eIFU for more information on use, indications, contraindications, warnings/precautions and availability within your market.
Contact us
Click the button below to get in touch. For more updates, follow us on X and LinkedIn. You can also view our VuMedi channel.