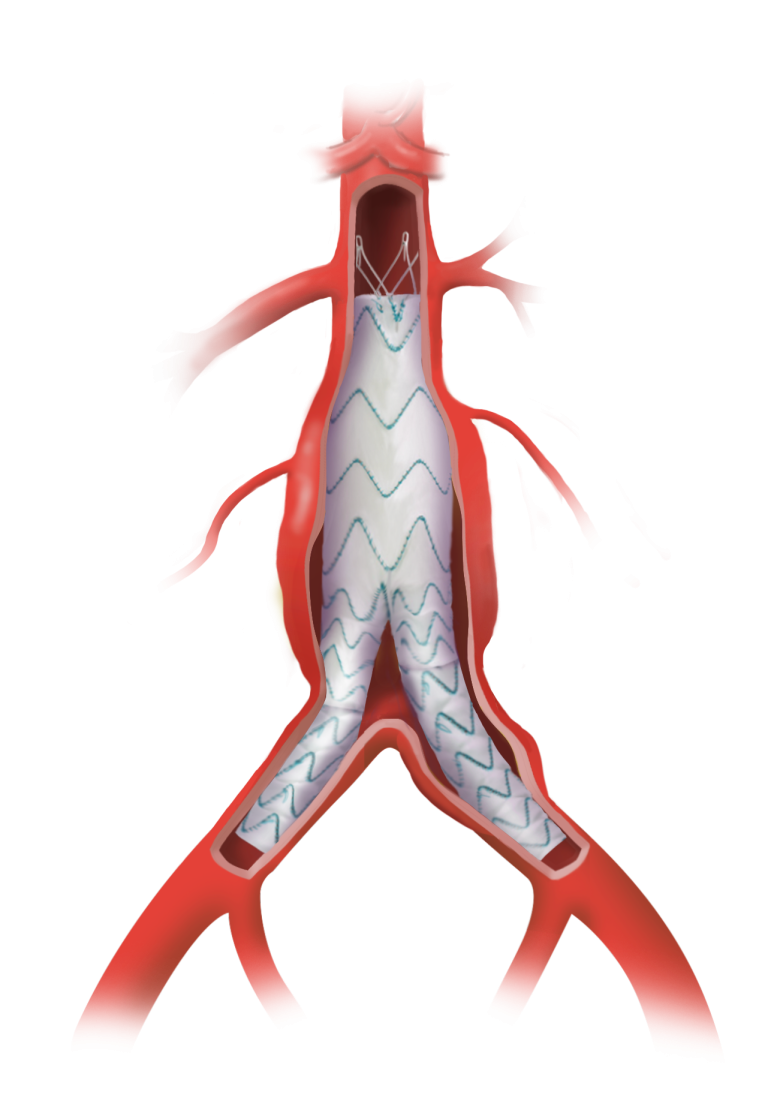
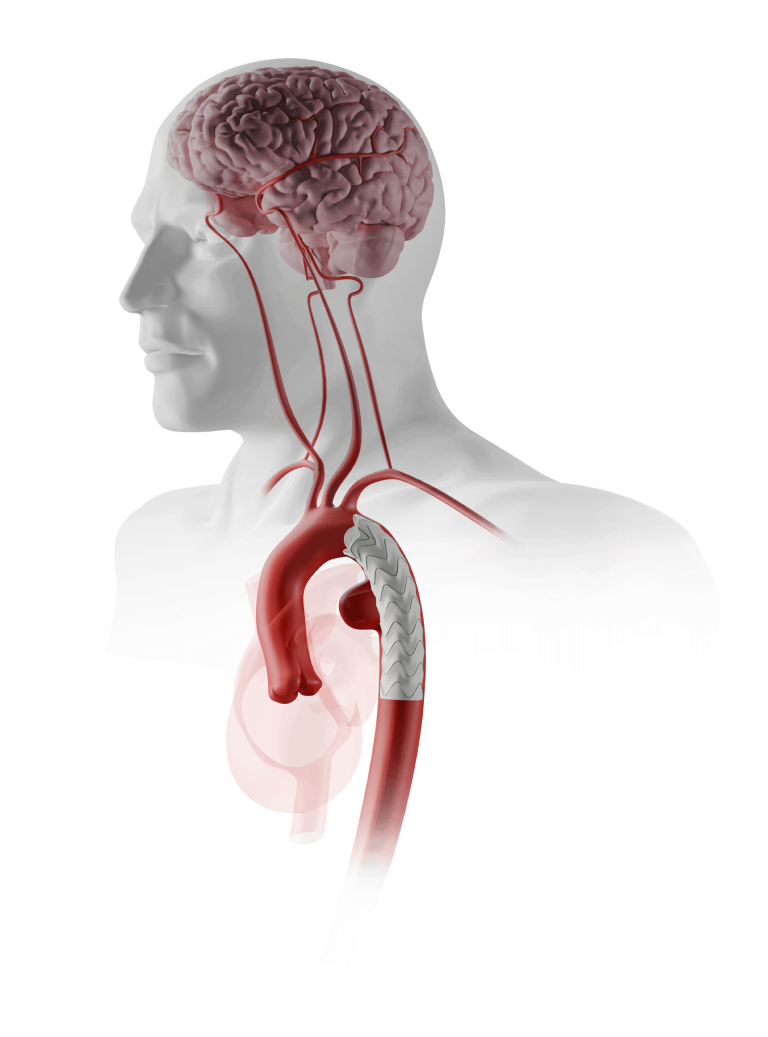
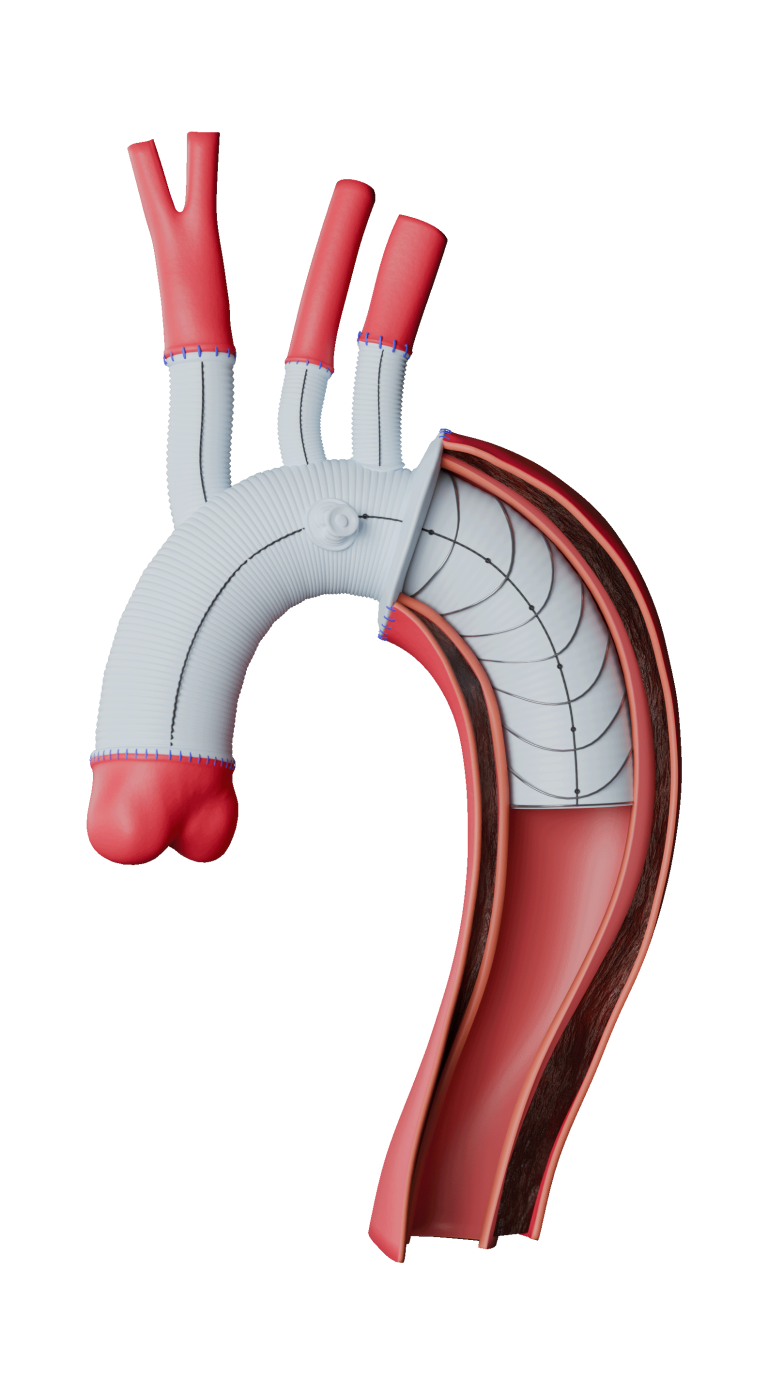
Custom TREO™ Fit
Where the Physician becomes the Tailor.
Infrarenal and Beyond…
Register your interest in the TREO Fit programme by clicking the button below.
Product Disclaimer
TREO Fit is available via the custom-made program, exclusively for use with the CE-Marked TREO Abdominal Stent-Graft System.
Custom made devices are specifically made in accordance with a written prescription of any person authorised by national law by virtue of that person’s professional qualifications; which gives (1) specific design characteristics provided under that person’s responsibility and (2) is intended for the sole use of a particular patient exclusively to meet their individual conditions and needs.
Custom made devices are not available in the US and availability is subject to local regulatory approval.
Instructions for Use
View IFU at eifu.terumoaortic.com for more information on use, indications, contraindications and warnings/precautions.
Contact us
Click the button below to get in touch. For more updates, follow us on X and LinkedIn. You can also view our VuMedi channel.