Powered By
Proprietary Technology
Our products are powered by technologies that help ensure that every product you use can be implanted and deployed safely.
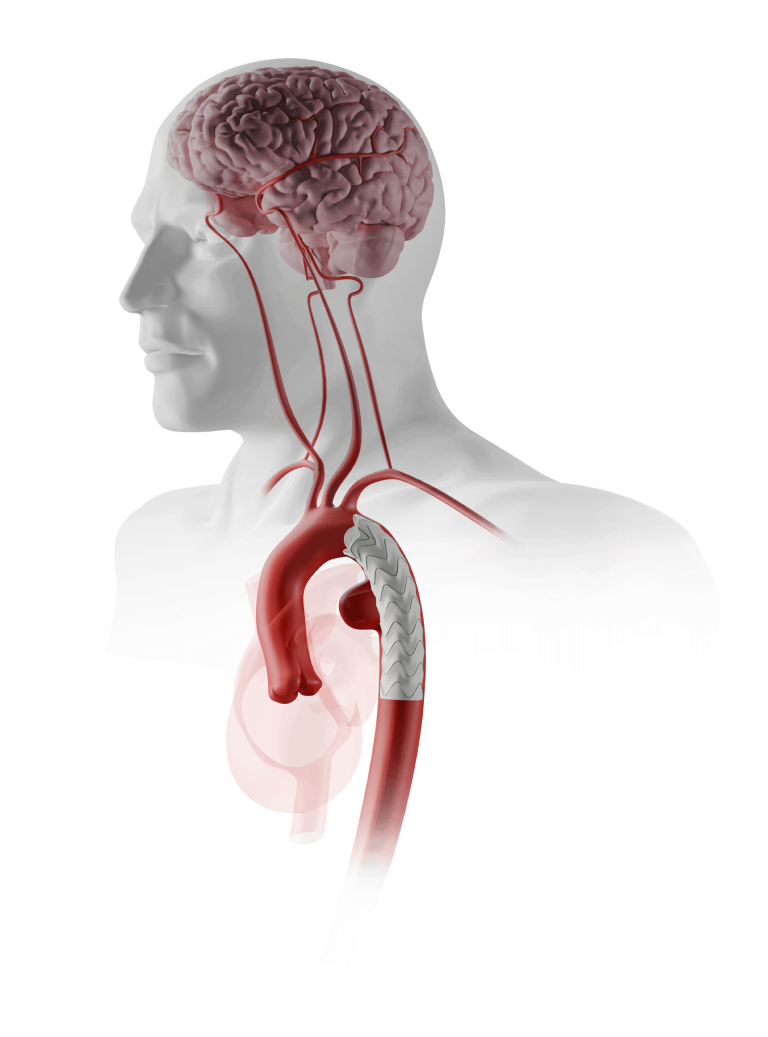
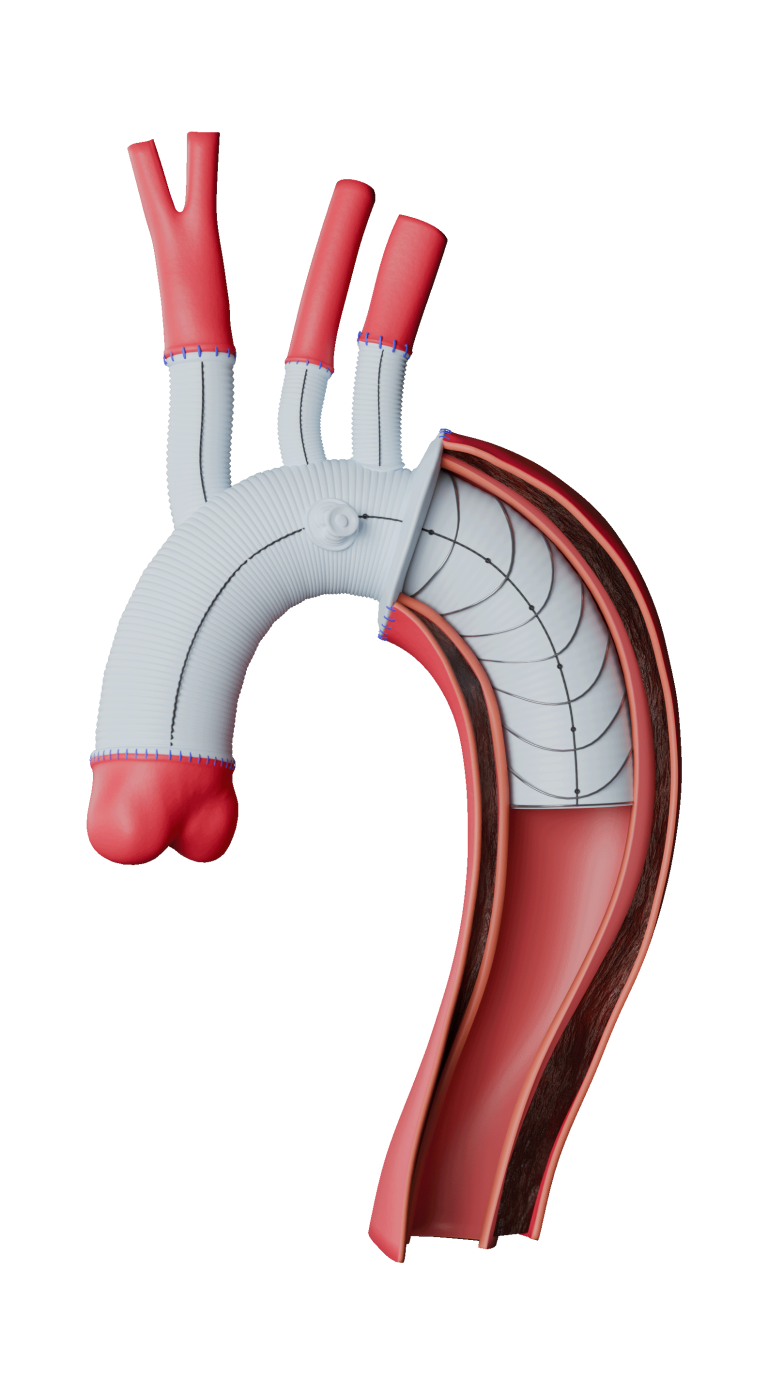
Advanced Delivery
Terumo Aortic delivery systems use proprietary technology to reposition, deploy and facilitate the implant and overall procedure.
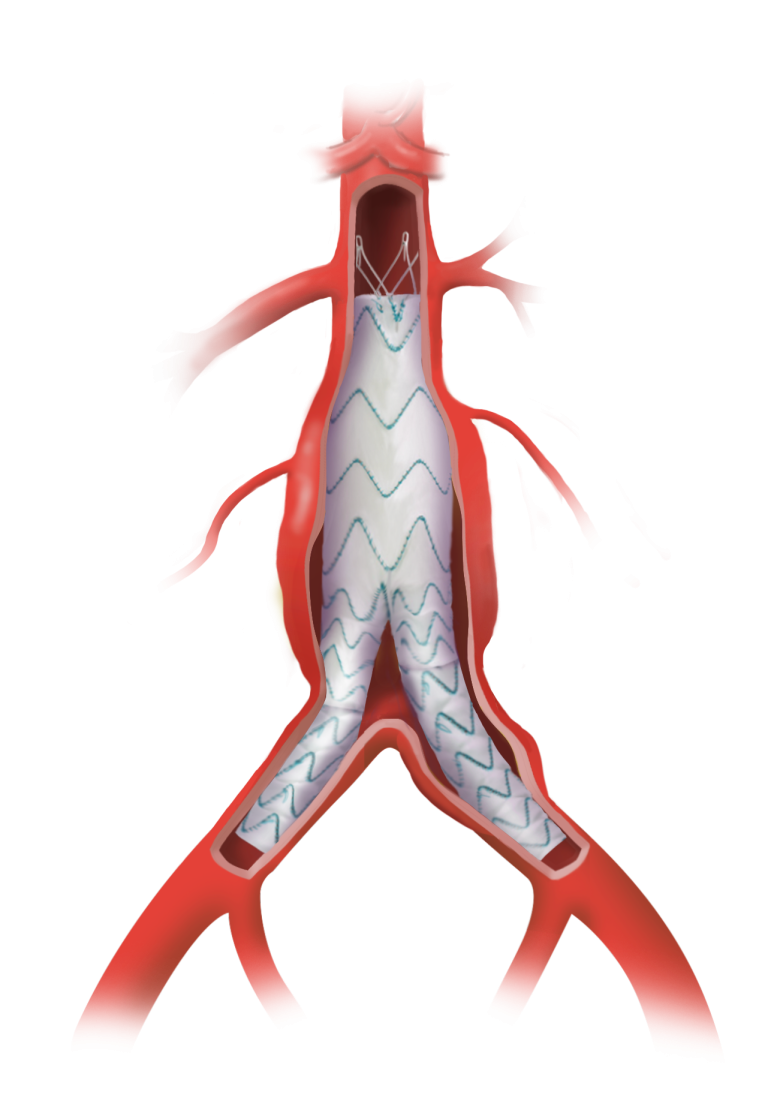
Knitting & Weaving
We utilise looms specially adapted to the needs of aortic repair textiles. Our unique Köper knitted structure features yarns arranged perpendicular to each other on the inner surface.
This preserves all the advantages of a knitted material, such as superb handling, absence of fraying and also displays significantly reduced dilatation when compared to warp-knitted grafts.1, 2
Sealant Technology
Sealed products feature a patented gelatin outer coating to assist the implantation process.
The gelatin technology is also an ideal sealant for bonding to the antibiotic rifampicin, which is used to minimise the occurrence of graft infection after implantation.†, 3, 4
Terumo Aortic’s gelatin sealed devices may be soaked with the anticoagulant Heparin or the antibiotic Rifampicin prior to implant.
Unlike other sealants, Terumo Aortic’s gelatin is broken down by the body’s own natural means over a period of approximately 14 days.This process does not provoke a prolonged inflammatory response within the patient’s body.5
This sealant removal rate allows natural tissue ingrowth to occur within the structure of the graft thereby mimicking the body’s natural mechanisms.6
References
Walker D et al. (1995). Novel structure for a polyester vascular prosthesis with improved mechanical properties. Society for Biomaterials.
Goëau-Brissonnière O. et al. (2000). Can knitting effect dilation of polyester bifurcated prosthesis? A randomised study with the use of helical computed tomographic scanning. Journal of Vascular Surgery. ;31:157-163.
Goëau-Brissonniere O et al. (1995). Rifampin Bonded Graft European Trial: (RBGET) Meeting.
Ashton TR et al. (1990). Antibiotic loading of vascular grafts. In: Proceedings of the 16th Annual Meeting of the Society for Biomaterials. Charleston, USA; 13.
Vohra R et al. (1987). Sealed versus unsealed knitted Dacron® prosthesis: A comparison of the acute phase protein response. Annals of Vasc Surg. I:548-551. doi: https://doi.org/10.1016/S0890-5096(06)61438-6.
Guidoin R, et al. (1987). In-Vitro and In-Vivo Characterization of an Impervious Polyester Arterial Prosthesis: The Gelseal Triaxial® Graft. Biomaterials. Vol 8 November.
Product Disclaimer
The rifampicin loading procedure is subject to local regulatory approval and has not been approved in the United States of America, Canada or Singapore.
An EU Declaration of Conformity may be requested from regulatoryaffairsuk@terumoaortic.com
Instructions for Use
View the eIFU for more information on use, indications, contraindications, warnings/precautions and availability within your market.
Careers at Terumo Aortic